Abstract
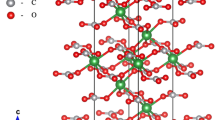
Magnesite (MgCO3), calcite (CaCO3), dolomite [(Ca, Mg)CO3], and siderite (FeCO3) are among the best-studied carbonate minerals at high pressures and temperatures. Although they all exhibit the calcite-type structure (\({\text{R}}\bar{3}{\text{c}}\)) at ambient conditions, they display very different behavior at mantle pressures. To broaden the knowledge of the high-pressure crystal chemistry of carbonates, we studied spherocobaltite (CoCO3), which contains Co2+ with cation radius in between those of Ca2+ and Mg2+ in calcite and magnesite, respectively. We synthesized single crystals of pure spherocobaltite and studied them using Raman spectroscopy and X-ray diffraction in diamond anvil cells at pressures to over 55 GPa. Based on single crystal diffraction data, we found that the bulk modulus of spherocobaltite is 128 (2) GPa and K′ = 4.28 (17). CoCO3 is stable in the calcite-type structure up to at least 56 GPa and 1200 K. At 57 GPa and after laser heating above 2000 K, CoCO3 partially decomposes and forms CoO. In comparison to previously studied carbonates, our results suggest that at lower mantle conditions carbonates can be stable in the calcite-type structure if the radius of the incorporated cation(s) is equal or smaller than that of Co2+ (i.e., 0.745 Å).










Similar content being viewed by others
References
Agilent (2014) CrysAlis PRO. Agilent Technologies Ltd, Yarnton
Angel JR, Alvaro M, Gonzalez-Platas J (2014) EosFit7c and a Fortran module (library) for equation of state calculations. Z Kristallogr Cryst Mat 229:405–419
Badro J, Brodholt JP, Piet H, Siebert J, Ryerson FJ (2015) Core formation and core composition from coupled geochemical and geophysical constraints. Proc Natl Acad Sci 112(40):12310–12314
Barton IF, Yang H, Barton MD (2014) The mineralogy, geochemistry, and metallurgy of cobalt in the rhombohedral carbonates. Can Mineral 52:653–670
Boulard E, Gloter A, Corgne A, Antonangeli D, Auzende AL, Perrillat JP, Guyot F, Fiquet G (2011) New host for carbon in deep Earth. PNAS 108:5184–5187
Boulard E, Pan D, Galli G, Liu Z, Mao W (2014) Tetrahedrally coordinated carbonates in Earth’s lower mantle. Nat Commun. doi:10.1038/ncomms7311
Boulard E, Goncharov AF, Blanchard M, Mao WL (2015) Pressure-induced phase transition in MnCO3 and its implications on the deep carbon cycle. J Geophys Res Solid Earth. doi:10.1002/2015JB011901
Bridgman PW (1939) The high pressure behavior of miscellaneous minerals. Am J Sci 237:7–18
Burns RG (1993) Mineralogical applications of crystal field theory. Cambridge University Press, Cambridge
Carr MH, Turekian KK (1960) The geochemistry of cobalt. Geochim Cosmochim Acta 23:9–60
Cerantola V, McCammon C, Kupenko I, Kantor I, Marini C, Wilke M, Ismailova L, Solopova N, Chumakov AI, Pascarelli S, Dubrovinsky L (2015) High-pressure spectroscopic study of siderite (FeCO3) with focus on spin crossover. Am Mineral 100:2670–2681
Effenberger H, Mereiter K, Zemann J (1981) Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithsonite and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Z Kristallogr 156:233–243
Farfan GA, Boulard E, Wang S, Mao WL (2013) Bonding and electronic changes in rhodochrosite at high pressure. Am Mineral 98:1817–1823
Farrugia LJ (2012) WinGX and ORTEP for windows: an update. J Appl Crystallogr 45:849–854
Fei Y, Ricolleau A, Frank M, Mibe K, Shen G, Prakapenka V (2007) Toward an internally consistent pressure scale. PNAS 104:9182–9186
Fiquet G, Guyot F, Itie JP (1994) High-pressure X-ray diffraction study of carbonates—MgCO3, CaMg(CO3)2, and CaCO3. Am Mineral 79:15–23
French BM (1971) Stability relations of siderite (FeCO3) in the system Fe–C–O. Am J Sci 27:37–78
Frost DJ, Poe BT, Tronnes RG, Liebske C, Duba A, Rubie DC (2004) A new large-volume multianvil system. Phys Earth Planet Inter 143–144:507–514
Gao J, Zhu F, Lai XJ, Huang R, Qin S, Chen DL, Liu J, Zheng LR, Wu X (2014) Compressibility of natural smithsonite ZnCO3 up to 50 GPa. High Press Res 34:89–99
Goldsmith JR, Northrop DA (1965) Subsolidus phase relations in the systems CaCO3–MgCO3–CoCO3 and CaCO3–MgCO3–NiCO3. J Geol 73:817–829
Gonzalez-Platas J, Alvaro M, Nestola F, Angel RJ (2016) EosFit7-GUI: a new GUI tool for equation of state calculations, analyses and teaching. J Appl Crystallogr 49:1377–1382
Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427:60–63
Jagoutz E, Palme H, Baddenhausen H, Blum K, Cendales M, Dreibus G, Spettel B, Lorenz V, Wänke H (1979) The abundances of major, minor and trace elements in the earth’s mantle as derived from primitive ultramafic rocks. Proc Lunar Planet Sci Conf 10:2031–2050
Kantor I, Prakapenka V, Kantor A, Dera P, Kurnosov A, Sinogeikin S, Dubrovinskaia N, Dubrovinsky L (2012) BX90: a new diamond anvil cell design for X-ray diffraction and optical measurements. Rev Sci Instrum. doi:10.1063/1.4768541
Katsura T, Tsuchida Y, Ito E, Yagi T, Utsumi W, Akimoto S (1991) Stability of magnesite under the lower mantle conditions. Proc Japan Acad Ser B 67:57–60
Kupenko I, Dubrovinsky L, Dubrovinskaia N, McCammon C, Glazyrin K, Bykova E, Boffa-Ballaran T, Sinmyo R, Chumakov A, Potapkin V, Kantor A, Rüffer R, Hanfland M, Crichton W, Merlini M (2012) Portable double-sided laser-heating system for Mössbauer spectroscopy and X-ray diffraction experiments at synchrotron facilities with diamond anvil cells. Rev Sci Instrum. doi:10.1063/1.4772458
Kurnosov A, Kantor I, Boffa-Ballaran T, Lindhardt S, Dubrovisnky L, Kuznetsov A, Zehnder BH (2008) A novel gas-loading system for mechanically closing of various types of diamond anvil cells. Rev Sci Instrum. doi:10.1063/1.2902506
Larson AC, von Dreele RB (1985) General structure analysis system (GSAS). Los Alamos National Laboratory Report, LAUR B6-748
Lavina B, Dera P, Downs RT, Prakapenka V, Rivers M, Sutton S, Nicol M (2009) Siderite at lower mantle conditions and the effects of the pressure-induced spin-pairing transition. GRL. doi:10.1029/2009GL039652
Lavina B, Dera P, Downs RT, Tschauner O, Yang W, Shebanova O, Shen G (2010a) Effect of dilution on the spin pairing transition in rhombohedral carbonates. High Press Res 30:224–229
Lavina B, Dera P, Downs RT, Yang W, Sinogeikin S, Meng Y, Shenand G, Schiferl D (2010b) Structure of siderite FeCO3 to 56 GPa and hysteresis of its spin-pairing transition. Phys Rev B. doi:10.1103/PhysRevB.82.064110
Lin JF, Liu J, Jacobs C, Prakapenka VB (2012) Vibrational and elastic properties of ferromagnesite across the electronic spin-pairing transition of iron. Am Mineral 97:583–591
Liu J, Lin J, Prakapenka VB (2015) High-pressure orthorhombic ferromagnesite as a potential deep-mantle carbon carrier. Sci Rep. doi:10.1038/srep07640
Liu J, Caracas R, Fan D, Bobocioiu E, Zhang D, Mao WL (2016) High-pressure compressibility and vibrational properties of (Ca, Mn)CO3. Am Min 101:2723–2730
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure Gauge to 800 kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–7676
Mao Z, Armentrout M, Rainey E, Manning CE, Dera P, Prakapenka VB, Kavner A (2011) Dolomite III: a new candidate lower mantle carbonate. GRL. doi:10.1029/2011GL049519
Mattila A, Rylkkänen T, Rueff JP, Huotari S, Vankó G, Hanfland M, Lehtinen M, Hämäläinen K (2007) Pressure induced magnetic transition in siderite FeCO3 studied by X-ray emission spectroscopy. J Phys Condens Matter. doi:10.1088/0953-8984/19/38/386206
Merlini M, Crichton WA, Hanfland M, Gemmi M, Müller H, Kupenko I, Dubrovinsky L (2012a) Structures of dolomite at ultrahigh pressure and their influence on the deep carbon cycle. PNAS 109:13509–13514
Merlini M, Hanfland M, Crichton WA (2012b) CaCO3-III and CaCO3-VI, high-pressure polymorphs of calcite: possible host structures for carbon in the Earth’s mantle. EPSL 333–334:265–271
Merlini M, Hanfland M, Gemmi M (2015) The MnCO3-II high-pressure polymorph of rhodochrosite. Am Mineral 100:2625–2629
Minch R, Seoung DH, Ehm L, Winkler B, Knorr K, Peters L, Borkowski LA, Parise JB, Lee Y, Dubrovinsky L, Depmeier W (2010) High-pressure behavior of otavite (CdCO3). J Alloys Compd 508:251–257
Ono S (2007) High-pressure phase transformation in MnCO3: a synchrotron XRD study. Mineral Mag 71:105–111
Pertlik F (1986) Structures of hydrothermally synthesized cobalt (II) carbonate and nickel (II) carbonate. Acta Cryst C 42:4–5
Reeder RJ (1983) Crystal chemistry of the rhombohedral carbonates. Rev Mineral 11:1–47
Rutt HN, Nicola JH (1974) Raman spectra of carbonates of calcite type. J Phys C Solid State Phys 7:4522–4528
Santillán J, Williams Q (2004) A high-pressure and X-ray study of FeCO3 and MnCO3: comparison with CaMg(CO3)2—dolomite. Phys Earth Planet Inter 143–144:291–304
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Crystallogr B 25:925
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Shi W, Fleet M, Shieh SR (2012) High-pressure phase transitions in Ca-Mn carbonates (Ca, Mn)CO3 studied by Raman spectroscopy. Am Mineral 97:999–1001
Suito K, Namba J, Horikawa T, Taniguchi Y, Sakurai N, Kobayashi M, Onodera A, Shimomura O, Kikegawa T (2001) Phase relations of CaCO3 at high pressure and high temperature. Am Mineral 86:997–1002
Taran MN, Langer K, Koch-Mueller M (2008) Pressure dependence of color of natural uvarovite: the barochromic effect. Phys Chem Miner 35:175–177
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–182
Veizer J (1983) Trace elements and isotopes in sedimentary carbonates. Rev Mineral 11:265–299
Vizgirda J, Ahrens TJ (1982) Shock compression of aragonite and implications for the equation of states of carbonates. J Geophys Res 87:4747–4758
Zhang J, Reeder RJ (1999) Comparative compressibilities of calcite-structure carbonates: deviations from empirical relations. Am Mineral 84:861–870
Acknowledgements
We thank the European Synchrotron Radiation Facility for provision of synchrotron radiation (ID09A) and Michael Hanfland for additional technical assistance. We also thank Tiziana Boffa-Ballaran for help with data analysis software and Alexander Kurnosov for the gas loading of diamond anvil cells. The project was supported by funds from the German Science Foundation (DFG) through the CarboPaT Research Unit FOR2125 (Mc3/20, Du393/9), the German Federal Ministry for Education (BMBF), and the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chariton, S., Cerantola, V., Ismailova, L. et al. The high-pressure behavior of spherocobaltite (CoCO3): a single crystal Raman spectroscopy and XRD study. Phys Chem Minerals 45, 59–68 (2018). https://doi.org/10.1007/s00269-017-0902-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-017-0902-5