Abstract
Background
Botulinum toxin type A (BoNT/A) has been used in aesthetic applications worldwide, including glabellar lines. Currently, four BoNT/A preparations were approved for the improvement of moderate-to-severe glabellar lines: onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, and prabotulinumtoxinA. DaxibotulinumtoxinA is a new form of BoNT/A drug that is developed in clinical application. We performed this network meta-analysis (NMA) to assess the efficacy and safety of all these different BoNT/A formulations for treating glabellar lines.
Methods
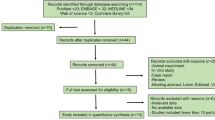
The investigators searched randomized controlled trials (RCTs) using the Medical Subject Headings (MeSH) terms “botulinum toxin” and “glabellar lines.” We searched the relevant studies in electronic databases as following: PubMed, Elsevier, EMBASE and the Cochrane Library. The end points included the percentage of subjects with a glabellar line severity (GLS) score of none (0) or mild (1), and the percentage of subjects achieving ≥ 1-point and 2-point improvement in glabellar line severity at maximum frown at approximately month 1 by the investigators’ assessment.
Results
All formulations of BoNT/A were far superior to placebo in efficacy. DaxibotulinumtoxinA was the only treatment that significantly increased the proportion of subjects achieving ≥ 1 point improvement in GLS score compared with other BoNT/A formulations. Moreover, daxibotulinumtoxinA was ranked the highest for the proportion of subjects achieving ≥ 2-point improvement in GLS score. No significant differences were revealed for the incidence of any adverse events (AEs) that related to treatment or drug among all BoNT/A preparations.
Conclusion
The overall results of this NMA suggested that daxibotulinumtoxinA is a new BoNT/A preparation that may be not only more effective but also well-tolerated for the treatment of glabellar lines.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.











Similar content being viewed by others
References
Kim BJ, Kwon HH, Park SY et al (2014) Double-blind, randomized non-inferiority trial of a novel botulinum toxin A processed from the strain CBFC26, compared with onabotulinumtoxin A in the treatment of glabellar lines. J Eur Acad Dermatol Venereol 28(12):1761–1767
Kawashima M, Harii K, Horiuchi Y et al (2020) Safety, efficacy, and patient satisfaction with onabotulinumtoxin A for the treatment of upper facial lines in Japanese subjects. Dermatol Surg 46(4):483–490
William Hanke C, Narins RS, Brandt F et al (2013) A randomized, placebo-controlled, double-blind phase iii trial investigating the efficacy and safety of incobotulinumtoxin A in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg 39(6):891–899
Yoo KH, Lee YW, Lee JS et al (2021) Efficacy and safety of a new botulinum toxin (HU-014) versus existing onabotulinumtoxin A in subjects with moderate to severe glabellar lines. Dermatol Surg 47(3):e91–e96
Gubanova E, Haddad TM, Bergerova Y et al (2018) Assessment of subject and physician satisfaction after long-term treatment of glabellar lines with AbobotulinumtoxinA (Dysport((R))/Azzalure((R))): primary results of the APPEAL noninterventional study. Aesthetic Plast Surg 42(6):1672–1680
Yoelin SG, Dhawan SS, Vitarella D et al (2018) Safety and efficacy of EB-001, a novel type E botulinum toxin, in subjects with glabellar frown lines: results of a phase 2, randomized, placebo-controlled, ascending-dose study. Plast Reconstr Surg 142(6):847e–855e
Ascher B, Rzany B, Kestemont P et al (2020) Liquid formulation of AbobotulinumtoxinA: A 6-month, phase 3, double-blind, randomized, placebo-controlled study of a single treatment, ready-to-use toxin for moderate-to-severe glabellar lines. Aesthetic Surg J 40(1):93–104
Polacco MA, Singleton AE, Barnes CH et al (2021) A double-blind, randomized clinical trial to determine effects of increasing doses and dose-response relationship of incobotulinumtoxin A in the treatment of glabellar rhytids. Aesthetic Surg J 41(6):500–511
Ogilvie P, Rivkin AZ, Dayan S et al (2019) Onabotulinumtoxin A for treatment of forehead and glabellar lines: subject-reported satisfaction and impact from a phase 3 double-blind study. Dermatol Surg 45(5):689–699
Kane MA, Gold MH, Coleman WR et al (2015) A randomized, double-blind trial to investigate the equivalence of incobotulinumtoxin A and onabotulinumtoxin A for glabellar frown lines. Dermatol Surg 41(11):1310–1319
Moers-Carpi M, Dirschka T, Feller-Heppt G et al (2012) A randomised, double-blind comparison of 20 units of onabotulinumtoxin A with 30 units of incobotulinumtoxin A for glabellar lines. J Cosmet Laser Ther 14(6):296–303
Schlessinger J, Cohen JL, Shamban A et al (2021) A multicenter study to evaluate subject satisfaction with two treatments of abobotulinumtoxin A a year in the glabellar lines. Dermatol Surg 47(4):504–509
Kaufman J, Cohen JL, Peredo MI et al (2019) Clinical assessment of 2 licensed abobotulinumtoxin A injection volumes for the treatment of glabellar lines. Dermatol Surg 45(10):1274–1284
Schlessinger J, Friedmann DP, Mayoral F et al (2021) Abobotulinumtoxin A treatment of glabellar lines using a new reconstitution and injection volume: randomized, Placebo-controlled data. J Drugs Dermatol 20(9):988–995
Kestemont P, Hilton S, Andriopoulos B et al (2022) Long-term efficacy and safety of liquid abobotulinumtoxina formulation for moderate-to-severe glabellar lines: a phase III, double-blind, randomized, placebo-controlled and open-label study. Aesthetic Surg J 42(3):301–313
Jones D, Carruthers J, Narins RS et al (2014) Efficacy of incobotulinumtoxin A for treatment of glabellar frown lines: a post hoc pooled analysis of 2 randomized, placebo-controlled, phase 3 trials. Dermatol Surg 40(7):776–785
Carruthers A, Carruthers J, Coleman WR et al (2013) Multicenter, randomized, phase III study of a single dose of incobotulinumtoxin A, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg 39(4):551–558
Sattler G, Callander MJ, Grablowitz D et al (2010) Noninferiority of incobotulinumtoxin A, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg 36(Suppl 4):2146–2154
Beer KR, Shamban AT, Avelar RL et al (2019) Efficacy and safety of prabotulinumtoxin A for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg 45(11):1381–1393
Rzany BJ, Ascher B, Avelar RL et al (2020) A multicenter, randomized, double-blind, placebo-controlled, single-dose, phase III, non-inferiority study comparing prabotulinumtoxin A and onabotulinumtoxin A for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J 40(4):413–429
Won CH, Kim HK, Kim BJ et al (2015) Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxin A in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol 54(2):227–234
Bertucci V, Humphrey S, Carruthers J et al (2017) Comparing injectable daxibotulinumtoxin A and onabotulinumtoxin A in moderate and severe glabellar lines: additional analyses from a phase 2, randomized, dose-ranging, double-blind, multicenter study. Dermatol Surg 43(Suppl 3):S262–S273
Guo Y, Lu Y, Liu T et al (2015) Efficacy and safety of botulinum toxin type A in the treatment of glabellar lines: a meta-analysis of randomized, Placebo-controlled, double-blind trials. Plast Reconstr Surg 136(3):310e–318e
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784
Higgins JPT (2020) Cochrane handbook for systematic reviews of interventions, 2nd edn. Springer, Berlin
Bai F, Li GG, Liu Q et al (2019) Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res 2019:2546161
Joseph J, Moradi A, Lorenc ZP et al (2021) Abobotulinumtoxin A for the treatment of moderate-to-severe glabellar lines: a randomized, dose-escalating, double-blind study. J Drugs Dermatol 20(9):980–987
Monheit GD, Baumann L, Maas C et al (2020) Efficacy, safety, and subject satisfaction after abobotulinumtoxin A treatment for moderate to severe glabellar lines. Dermatol Surg 46(1):61–69
Brandt F, Swanson N, Baumann L et al (2009) Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg 35(12):1893–1901
Ascher B, Kestemont P, Boineau D et al (2018) Liquid formulation of Abobotulinumtoxin A exhibits a favorable efficacy and safety profile in moderate to severe glabellar lines: a randomized, double-blind, placebo- and active comparator-controlled trial. Aesthetic Surg J 38(2):183–191
Hanke CW, Narins RS, Brandt F et al (2013) A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxin A in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg 39(6):891–899
Rzany BJ, Ascher B, Avelar RL et al (2020) A multicenter, randomized, double-blind, placebo-controlled, single-dose, phase III, non-inferiority study comparing prabotulinumtoxin A and onabotulinumtoxin A for the treatment of moderate to severe glabellar lines in adult patients. Aesthetic Surg J 40(4):413–429
Carruthers JD, Fagien S, Joseph JH et al (2017) Injectable daxibotulinumtoxina for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxina and placebo. Dermatol Surg 43(11):1321–1331
Carruthers JD, Fagien S, Joseph JH et al (2020) Daxibotulinumtoxin A for injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg 145(1):45–58
Kerscher M, Fabi S, Fischer T et al (2021) Incobotulinumtoxin A demonstrates safety and prolonged duration of effect in a dose-ranging study for glabellar lines. J Drugs Dermatol JDD 20(10):1052–1060
Fabi SG, Cohen JL, Green LJ et al (2021) Daxibotulinumtoxin A for injection for the treatment of glabellar lines: efficacy results from SAKURA 3, a large, open-label, phase 3 safety study. Dermatol Surg 47(1):48–54
Glogau R, Kontis TC, Liu Y et al (2021) Progressive improvement in static glabellar lines after repeated treatment with daxibotulinumtoxina for injection. Dermatol Surg 47(12):1579–1584
Bertucci V, Solish N, Kaufman-Janette J et al (2020) DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J Am Acad Dermatol 82(4):838–845
Funding
This work is supported by the Medical Health Science and Technology Project of Shandong Provincial Health Commission (Grant Number 202104120294).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Sui, C., **a, X. et al. Efficacy and Safety of Botulinum Toxin Type A for Treatment of Glabellar Lines: A Network Meta-Analysis of Randomized Controlled Trials. Aesth Plast Surg 47, 365–377 (2023). https://doi.org/10.1007/s00266-022-03018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-022-03018-y