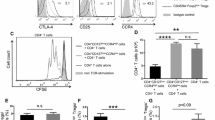
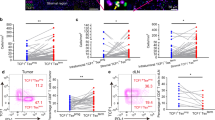
Abstract
Cancer immunotherapy using immune checkpoint inhibitors (ICIs) has been recognized as a novel therapeutic option for head and neck squamous cell carcinoma (HNSCC). However, only approximately 20–30% of patients with recurrent/metastatic (R/M) HNSCC benefit. Moreover, the mechanisms underlying the response to ICIs remain unclear. We investigated the proportion, activation status, and expression level of immune checkpoint molecules in circulating T cell subsets in R/M HNSCC patients treated with nivolumab using flow cytometry and mass cytometry, and then determined whether treatment response was associated with these values. We also assessed the changes in the frequency of tumor-associated antigens, MAGE-A4 and p53, -specific T cells prior to and after nivolumab treatment using the IFN-γ ELISPOT assay. The proportion of activated CD4+ and CD8+ TEMRA cells significantly increased in the disease-controlled patients but not in disease-progressed patients. As expected, the expression of PD-1 in T cells markedly decreased regardless of the therapeutic response. Meanwhile, T cell immunoglobulin mucin-3 expression on CD8+ T cells was significantly higher in patients with disease progression than in disease-controlled patients after treatment. The frequency of the tumor-associated antigens, MAGE-A4- and p53-specific T cells, was not correlated with clinical responses; however, in the disease-controlled patients, the frequency of MAGE-A4-specific T cells was significantly augmented. We concluded that in R/M HNSCC patients treated with nivolumab, circulating T cells show dynamic alterations depending on treatment efficacy. An analysis of the immunokinetics of circulating T cells could thus provide new insights into rational therapeutic strategies in cancer immunotherapy for HNSCC.





Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- CyTOF:
-
Cytometry by time-of-flight
- DAB:
-
3,3’-Diaminobenzidine
- ELISPOT:
-
Enzyme-linked immunosorbent spot
- FCS:
-
Fetal calf serum
- Foxp3:
-
Forkhead box P3
- HNSCC:
-
Head and neck squamous cell carcinoma
- IFN:
-
Interferon
- IHC:
-
Immunohistochemistry
- ICI:
-
Immune checkpoint inhibitor
- IL:
-
Interleukin
- Lag-3:
-
Lymphocyte activation gene 3
- MAGE-A4:
-
Melanoma-associated antigen-A4
- MDS:
-
Multi-dimensional scaling
- PBMC:
-
Peripheral blood mononuclear cell
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-1 ligand 1
- PHA:
-
Phytohemagglutinin
- R/M:
-
Recurrent/metastatic
- SFC:
-
Spot-forming-cells
- TAA:
-
Tumor-associated antigen
- TCM:
-
Central memory T cells
- TEM:
-
Effector memory T cells
- TEMRA:
-
Terminally differentiated effector memory T cells
- Tim-3:
-
T cell immunoglobulin mucin-3
- TN:
-
Naïve T cells
- UMAP:
-
Uniform manifold approximation and projection
References
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15:486–499
Thommen DS, Schumacher TN (2018) T cell dysfunction in cancer. Cancer Cell 33:547–562
Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL (2015) Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 42:587–600
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394:1915–1928
Cramer JD, Burtness B, Ferris RL (2019) Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol 99:104460
Bai R, Lv Z, Xu D, Cui J (2020) Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 8:34
Otoshi T, Nagano T, Tachihara M, Nishimura Y (2019) Possible biomarkers for cancer immunotherapy. Cancers (Basel) 11:935
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50:1–11
Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10
Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, Fleener C (2019) Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer 7:325
Hernandez C, Arasanz H, Chocarro L, Bocanegra A, Zuazo M, Fernandez-Hinojal G et al (2020) Systemic blood immune cell populations as biomarkers for the outcome of immune checkpoint inhibitor therapies. Int J Mol Sci 21:2411
Valpione S, Galvani E, Tweedy J, Mundra PA, Banyard A, Middlehurst P et al (2020) Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer 1:210–221
Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A et al (2017) Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114:4993–4998
Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C et al (2018) Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol 9:1613
Haddad R, Concha-Benavente F, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD et al (2019) Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: a subgroup analysis of a randomized phase 3 clinical trial. Cancer 125:3208–3218
von Witzleben A, Wang C, Laban S, Savelyeva N, Ottensmeier CH (2020) HNSCC: Tumour antigens and their targeting by immunotherapy. Cells 9:2103
Watling DL, Gown AM, Coltrera MD (1992) Overexpression of p53 in head and neck cancer. Head Neck 14:437–444
Nowicka M, Krieg C, Crowell HL, Weber LM, Hartmann FJ, Guglietta S et al (2017) CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 6:748
Yamada K, Masuda K, Ida S, Tada H, Bando M, Abe K et al (2021) In vitro assessment of antitumor immune responses using tumor antigen proteins produced by transgenic silkworms. J Mater Sci Mater Med 32:58
Motokawa Y, Kokubo M, Kuwabara N, Tatematsu KI, Sezutsu H, Takahashi H et al (2018) Melanoma antigen family A4 protein produced by transgenic silkworms induces antitumor immune responses. Exp Ther Med 15:2512–2518
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712
Sallusto F, Geginat J, Lanzavecchia A (2004) Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763
Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA (2018) The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol Rev 283:194–212
Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G et al (2017) Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun 8:1473
Kunert A, Basak EA, Hurkmans DP, Balcioglu HE, Klaver Y, van Brakel M et al (2019) CD45RA+CCR7- CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. J Immunother Cancer 7:149
Pirozyan MR, McGuire HM, Emran AA, Tseng HY, Tiffen JC, Lee JH et al (2020) Pretreatment innate cell populations and CD4 T cells in blood are associated with response to immune checkpoint blockade in melanoma patients. Front Immunol 11:372
Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T et al (2018) Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 3:e59125
Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH et al (2019) The first-week proliferative response of peripheral blood PD-1+CD8+ T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res 25:2144–2154
Wu P, Zhao L, Chen Y, **n Z, Lin M, Hao Z et al (2021) CD38 identifies pre-activated CD8+ T cells which can be reinvigorated by anti-PD-1 blockade in human lung cancer. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-021-02949-w
Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O et al (2019) Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med 216:2128–2149
Puntigam LK, Jeske SS, Götz M, Greiner J, Laban S, Theodoraki MN et al (2020) Immune checkpoint expression on immune cells of HNSCC patients and modulation by chemo- and immunotherapy. Int J Mol Sci 21:5181
Ding G, Shen T, Yan C, Zhang M, Wu Z, Cao L (2019) IFN-γ down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer 19:1053
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG et al (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7:10501
Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL (2017) Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 6:e1261779
Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM et al (2021) Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res 27:3620–3629
Merhi M, Raza A, Inchakalody VP, Nashwan AJJ, Allahverdi N, Krishnankutty R et al (2018) Squamous cell carcinomas of the head and neck cancer response to programmed cell death Protein-1 targeting and differential expression of immunological markers: a case report. Front Immunol 9:1769
Veatch JR, Singhi N, Jesernig B, Paulson KG, Zalevsky J, Iaccucci E et al (2020) Mobilization of pre-existing polyclonal T cells specific to neoantigens but not self-antigens during treatment of a patient with melanoma with bempegaldesleukin and nivolumab. J Immunother Cancer 8:e001591
Łuksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A et al (2017) A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551:517–520
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK et al (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351:1463–1469
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (Grant No. 20K18243 to Hideyuki T., No. 20K09747 to M.S., No. 19K18794 to S.I., No 19K18758 to I.M., and No. 20H03834 to K.C.) from the Ministry of Education, Culture, Sports, Science and Technology; a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Research Project for Sericultural Revolution), Japan; and Financial Support for the Promotion of University-Industry Collaborative Research Based on Regulatory Sciences at Gunma University.
Author information
Authors and Affiliations
Contributions
Hideyuki T. and KC were involved in conception and design. Hiroe T., Hideyuki T., KY, KM, YN, MU, MS, SI, IM, TM, KT and HS were involved in sample preparation and data acquisition. Hiroe T., Hideyuki T., TO, ST and KC were involved in analysis and interpretation of data. Hiroe T. and KC were involved in writing of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Ethical approval
This study was approved by the Ethical Committee of Gunma University Hospital (HS2017-152).
Informed consent
Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tada, H., Takahashi, H., Yamada, K. et al. Dynamic alterations of circulating T lymphocytes and the clinical response in patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Immunol Immunother 71, 851–863 (2022). https://doi.org/10.1007/s00262-021-03042-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03042-y