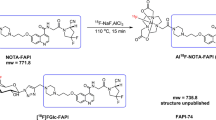
Abstract
Purpose
Fibroblast-activated protein (FAP) is highly expressed in cancer-associated fibroblasts (CAFs) of many solid cancers, but low or absent in normal tissues. Our study aimed to develop a novel FAP-specific tracer, namely [18F]FAP-2286, and evaluated its performance in comparison with well-established agents such as [18F]FAPI-42 and [68Ga]Ga-FAP-2286 in preclinical research, as well as 2-[18F]FDG in pilot clinical study.
Methods
[18F]FAP-2286 was manually synthesized in accordance with Good Manufacturing Practice (GMP). Subsequent investigations encompassed cell uptake, competitive binding affinity, internalization and efflux assays using HT-1080hFAP cell lines. PET imaging and biodistribution studies were conducted in HEK-293ThFAP, A549hFAP, HT-1080hFAP tumor-bearing mice as well as HEK-293T, A549 and HT-1080 control groups. Furthermore, clinical evaluation of [18F]FAP-2286 was performed in fifteen patients with various cancers compared to 2-[18F]FDG PET.
Results
The radiolabeling yield of [18F]FAP-2286 was 30.53 ± 5.20%, with a radiochemical purity exceeding 97%. In cell assays, [18F]FAP-2286 showed specific uptake, high internalization fraction and low cellular efflux. Rapid tumor uptake and satisfactory tumor retention was observed on micro-PET imaging and cancer patients. Meanwhile, the clinical research demonstrated that [18F]FAP-2286 may represent an alternative for low glucose-metabolism malignant tumors PET imaging such as gastric cancers.
Conclusion
[18F]FAP-2286 showed superior imaging quality including rapid and high target uptake and satisfactory retention in both tumor-bearing mice and cancer patients. It may emerge as a promising candidate for early or delayed phase imaging and 2-[18F]FDG non-avid cancers PET scan.






Similar content being viewed by others
Data availability
All data are included in this published article and its supplementary information file.
References
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39:783–3.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–8.
Teichgräber V, Monasterio C, Chaitanya K, Boger R, Gordon K, et al. Specific inhibition of fibroblast activation protein (FAP)-alpha prevents tumor progression in vitro. Adv Med Sci. 2015;60:264–2.
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–5.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9.
Wu F, Yang J, Liu J, Wang Y, Mu J, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218.
Zang J, Lin R, Wen X, Wang C, Zhao T, et al. A head-to-head comparison of 68Ga-LNC1007 and 2-18F-FDG/68Ga-FAPI-02 PET/CT in patients with various cancers. Clin Nucl Med. 2023;48:861–8.
Lin R, Lin Z, Chen Z, Zheng S, Zhang J, et al. [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of gastric cancer: comparison with [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2022;49:2960–1.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–2.
Glatting FM, Hoppner J, Kauczor HU, Huber PE, Kratochwil C et al. Subclass analysis of malignant, inflammatory and degenerative pathologies based on multiple timepoint FAPI-PET acquisitions using FAPI-02, FAPI-46 and FAPI-74. Cancers (Basel). 2022; 14.
Rosenkrans ZT, Massey CF, Bernau K, Ferreira CA, Jeffery JJ, et al. [68Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur J Nucl Med Mol Imaging. 2022;49:3705–6.
Zboralski D, Hoehne A, Bredenbeck A, Schumann A, Nguyen M, et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 2022;49:3651–7.
Pang Y, Zhao L, Meng T, Xu W, Lin Q, et al. PET imaging of fibroblast activation protein in various types of Cancer using 68Ga-FAP-2286: comparison with 18F-FDG and 68Ga-FAPI-46 in a single-center, prospective study. J Nucl Med. 2023;64:386–4.
Martiniova L, Palatis L, Etchebehere E, Ravizzini G. Gallium-68 in medical imaging. Curr Radiopharm. 2016;9:187–7.
Sahnoun S, Conen P, Mottaghy FM. The battle on time, money and precision: Da[18F] id vs. [68Ga]liath. Eur J Nucl Med Mol Imaging. 2020;47:2944–6.
Bernard-Gauthier V, Lepage ML, Waengler B, Bailey JJ, Liang SH, et al. Recent advances in 18F Radiochemistry: a Focus on B-18F, Si-18F, Al-18F, and C-18F Radiofluorination via spirocyclic iodonium ylides. J Nucl Med. 2018;59:568–2.
Wei Y, Zheng J, Ma L, Liu X, Xu S, et al. [18F]AlF-NOTA-FAPI-04: FAP-targeting specificity, biodistribution, and PET/CT imaging of various cancers. Eur J Nucl Med Mol Imaging. 2022;49:2761–73.
Yang T, Peng L, Qiu J, He X, Zhang D, et al. A radiohybrid theranostics ligand labeled with fluorine-18 and lutetium-177 for fibroblast activation protein-targeted imaging and radionuclide therapy. Eur J Nucl Med Mol Imaging. 2023;50:2331–1.
Wang S, Zhou X, Xu X, Ding J, Liu S, et al. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2021;48:4259–1.
Kuyumcu S, Kovan B, Sanli Y, Buyukkaya F, Has Simsek D, et al. Safety of fibroblast activation protein-targeted radionuclide therapy by a low-dose dosimetric approach using 177Lu-FAPI04. Clin Nucl Med. 2021;46:641–6.
Liu Y, Watabe T, Kaneda-Nakashima K, Shirakami Y, Naka S, et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging. 2022;49:871–0.
Hu K, Li J, Wang L, Huang Y, Li L, et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm Sin B. 2022;12:867–5.
Goh JB, Ng SK. Impact of host cell line choice on glycan profile. Crit Rev Biotechnol. 2018;38:851–7.
Fang J, Feng L, Meng L, Wang X, Liu H, et al. A novel 18F-labeled agonist for PET imaging of stimulator of interferon gene expression in tumor-bearing mice. Eur J Nucl Med Mol Imaging. 2022;50:27–7.
Giesel FL, Adeberg S, Syed M, Lindner T, Jiménez-Franco LD, et al. FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga labeling: biodistribution, radiation dosimetry, and tumor delineation in lung cancer patients. J Nucl Med. 2021;62:201–7.
Hu K, Wang L, Wu H, Huang S, Tian Y, et al. [18F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging. 2022;49:2833–3.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–6.
Filik M, Kir KM, Aksel B, Soyda Ç, Özkan E, et al. The role of 18F-FDG PET/CT in the primary staging of gastric cancer. Mol Imaging Radionucl Ther. 2015;24:15–0.
Kitajima K, Nakajo M, Kaida H, Minamimoto R, Hirata K, et al. Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci. 2017;79:527–3.
Qin C, Shao F, Gai Y, Liu Q, Ruan W, et al. 68Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with 18F-FDG PET/CT. J Nucl Med. 2022;63:81–8.
Acknowledgements
We gratefully appreciate all of the staff from the Department of Nuclear Medicine, the Sixth Affiliated Hospital and the First Affiliated Hospital of Guangzhou Medical University, for their contributions to this study. Finally, we thank to Prof. Kongzhen Hu for HEK-293ThFAP and A549hFAP cell lines.
Funding
This research was supported in part by the National Natural Science Foundation of China (No.81901772 and No.82001879), the Natural Science Foundation of Guangdong Province (No.2019A1515011893 and 2023A1515011300), the Basic and Applied Basic Research Foundation of Guangdong Province (No. 2020A1515110159), the Science and Technology Project of Guangzhou City (No. 202102010354), the State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia & The First People’s Hospital of Kashi Fund (No.SKL-HIDCA-2020-KS2) and the Zhong Nanshan Medical Foundation of Guangdong Province (ZNSA-2020003).
Author information
Authors and Affiliations
Contributions
Conceptualization, design and methodology: Shaoyu Liu, Zhanwen Zhang and **nlu Wang; experiments, data collection and analysis and writing the manuscript: Lifang Liu; part of the biodistribution and radiopharmaceutical preparation: Jiawei Zhong, Ziqi Zhang, **aoting Ye; funding acquisition: Zhanwen Zhang and Shaoyu Liu; revision of the article: all of the authors.
Corresponding authors
Ethics declarations
Ethics approval
All animal studies were performed according to Guangzhou Medical University Institutional Animal Care and Use Committee (No. 20230330). The clinical translational study was approved by the ethics committee of The Sixth Affiliated Hospital of Sun Yat-sen University (2023ZSLYEC-597). All procedures were conducted according to the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
Authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Zhong, J., Zhang, Z. et al. Preclinical study and first-in-human imaging of [18F]FAP-2286, and comparison with 2-[18F]FDG PET/CT in various cancer patients. Eur J Nucl Med Mol Imaging 51, 2012–2022 (2024). https://doi.org/10.1007/s00259-024-06626-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06626-9