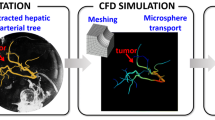
Abstract
Purpose
Transarterial radioembolization (TARE) procedures treat liver tumors by injecting radioactive microspheres into the hepatic artery. Currently, there is a critical need to optimize TARE towards a personalized dosimetry approach. To this aim, we present a novel microsphere dosimetry (MIDOS) stochastic model to estimate the activity delivered to the tumor(s), normal liver, and lung.
Methods
MIDOS incorporates adult male/female liver computational phantoms with the hepatic arterial, hepatic portal venous, and hepatic venous vascular trees. Tumors can be placed in both models at user discretion. The perfusion of microspheres follows cluster patterns, and a Markov chain approach was applied to microsphere navigation, with the terminal location of microspheres determined to be in either normal hepatic parenchyma, hepatic tumor, or lung. A tumor uptake model was implemented to determine if microspheres get lodged in the tumor, and a probability was included in determining the shunt of microspheres to the lung. A sensitivity analysis of the model parameters was performed, and radiation segmentectomy/lobectomy procedures were simulated over a wide range of activity perfused. Then, the impact of using different microspheres, i.e., SIR-Sphere®, TheraSphere®, and QuiremSphere®, on the tumor-to-normal ratio (TNR), lung shunt fraction (LSF), and mean absorbed dose was analyzed.
Results
Highly vascularized tumors translated into increased TNR. Treatment results (TNR and LSF) were significantly more variable for microspheres with high particle load. In our scenarios with 1.5 GBq perfusion, TNR was maximum for TheraSphere® at calibration time in segmentectomy/lobar technique, for SIR-Sphere® at 1–3 days post-calibration, and regarding QuiremSphere® at 3 days post-calibration.
Conclusion
This novel approach is a decisive step towards develo** a personalized dosimetry framework for TARE. MIDOS assists in making clinical decisions in TARE treatment planning by assessing various delivery parameters and simulating different tumor uptakes. MIDOS offers evaluation of treatment outcomes, such as TNR and LSF, and quantitative scenario-specific decisions.





Similar content being viewed by others
Data Availability
The model employed and data utilized to show results in this paper are available upon reasonable request from the corresponding author, subject to privacy/ethical considerations.
References
Bastiaannet R, Kappadath SC, Kunnen B, Braat AJAT, Lam MGEH, de Jong HWAM. The physics of radioembolization. EJNMMI Phys. 2018;5:22.
Kim SP, Cohalan C, Kopek N, Enger SA. A guide to 90Y radioembolization and its dosimetry. Phys Med. 2019;68:132–45.
Gulec SA, McGoron AJ. Radiomicrosphere dosimetry: principles and current state of the art. Semin Nucl Med. 2022;52:215–28.
Knight GM, Gordon AC, Gates V, Talwar A, Riaz A, Salem R, et al. Evolution of personalized dosimetry for radioembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2023;34:1214–25.
Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE, Loehr SP, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–25.
Malhotra A, Liu DM, Talenfeld AD. Radiation segmentectomy and radiation lobectomy: a practical review of techniques. Tech Vasc Interv Radiol. 2019;22:49–57.
Westcott MA, Coldwell DM, Liu DM, Zikria JF. The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Adv Radiat Oncol. 2016;1:351–64.
Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49:1682–99.
Reinders MTM, Smits MLJ, van Roekel C, Braat AJAT. Holmium-166 microsphere radioembolization of hepatic malignancies. Semin Nucl Med. 2019;49:237–43.
Ilhan H, Goritschan A, Paprottka P, Jakobs TF, Fendler WP, Todica A, et al. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J Nucl Med. 2015;56:1654–60.
Haste P, Tann M, Persohn S, LaRoche T, Aaron V, Mauxion T, et al. Correlation of technetium-99m macroaggregated albumin and yttrium-90 glass microsphere biodistribution in hepatocellular carcinoma: a retrospective review of pretreatment single photon emission CT and posttreatment positron emission tomography/CT. J Vasc Interv Radiol. 2017;28:722-730.e1.
Bertolet A, Wehrenberg-Klee E, Bobic M, Grassberger C, Perl J, Paganetti H, et al. Pre- and post-treatment image-based dosimetry in 90Y-microsphere radioembolization using the TOPAS Monte Carlo toolkit. Phys Med Biol. 2021;66:244002.
Villalobos A, Cheng B, Wagstaff W, Sethi I, Bercu Z, Schuster DM, et al. Tumor-to-normal ratio relationship between planning technetium-99 macroaggregated albumin and posttherapy yttrium-90 bremsstrahlung SPECT/CT. J Vasc Interv Radiol. 2021;32:752–60.
Young S, Chen T, Flanagan S, Golzarian J, Sanghvi T. Realized tumor to normal ratios in hepatocellular carcinoma patients undergoing transarterial radioembolization: a retrospective evaluation. Eur Radiol. 2022;32:4160–7.
Villalobos A, Arndt L, Cheng B, Dabbous H, Loya M, Majdalany B, et al. Yttrium-90 radiation segmentectomy of hepatocellular carcinoma: a comparative study of the effectiveness, safety, and dosimetry of glass vs. resin-based microspheres. J Vasc Interv Radiol. 2023;34:1226–34.
Pasciak AS, Abiola G, Liddell RP, Crookston N, Besharati S, Donahue D, et al. The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. Eur J Nucl Med Mol Imaging. 2020;47:816–27.
Maxwell AWP, Mendoza HG, Sellitti MJ, Camacho JC, Deipolyi AR, Ziv E, et al. Optimizing 90Y particle density improves outcomes after radioembolization. Cardiovasc Intervent Radiol. 2022;45:958–69.
Boas FE, Maxwell AWP. Beyond mean tumor dose: the importance of particle density in radioembolization. J Vasc Interv Radiol. 2023;34:1235–6.
Aramburu J, Antón R, Rodríguez-Fraile M, Sangro B, Bilbao JI. Computational fluid dynamics modeling of liver radioembolization: a review. Cardiovasc Intervent Radiol Springer. 2022;45:12–20.
Toskich B, Lewandowski RJ. Computational modeling of radioembolization: how to calculate infinity. Cardiovasc Intervent Radiol. Springer; 2021. pp. 2020–1.
Miller SR, Jernigan SR, Abraham RJ, Buckner GD. Comparison of bolus versus dual-syringe administration systems on glass yttrium-90 microsphere deposition in an in vitro microvascular hepatic tumor model. J Vasc Interv Radiol. 2023;34:11–20.
Crookston NR, Fung GSK, Frey EC. Development of a customizable hepatic arterial tree and particle transport model for use in treatment planning. IEEE Trans Radiat Plasma Med Sci. 2019;3:31–7.
Walrand S, Hesse M, Chiesa C, Lhommel R, Jamar F. The low hepatic toxicity per gray of 90Y glass microspheres is linked to their transport in the arterial tree favoring a nonuniform trap** as observed in posttherapy PET imaging. J Nucl Med. 2014;55:135–40.
Högberg J, Rizell M, Hultborn R, Svensson J, Henrikson O, Mölne J, et al. Simulation model of microsphere distribution for selective internal radiation therapy agrees with observations. Int J Radiat Oncol Biol Phys. 2016;96:414–21.
Pasciak AS, Bourgeois AC, Bradley YC. A microdosimetric analysis of absorbed dose to tumor as a function of number of microspheres per unit volume in 90Y Radioembolization. J Nucl Med. 2016;57:1020–6.
Correa-Alfonso CM, Withrow JD, Domal SJ, **ng S, Shin J, Grassberger C, et al. A mesh-based model of liver vasculature: implications for improved radiation dosimetry to liver parenchyma for radiopharmaceuticals. EJNMMI Phys. 2022;9:28.
Lewandowski RJ, Minocha J, Memon K, Riaz A, Gates VL, Ryu RK, et al. Sustained safety and efficacy of extended-shelf-life 90Y glass microspheres: long-term follow-up in a 134-patient cohort. Eur J Nucl Med Mol Imaging. 2014;41:486–93.
Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90 Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47.
Campbell AM, Bailey IH, Burton MA. Analysis of the distribution of intra-arterial microspheres in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2000;45:1023–33.
Högberg J, Rizell M, Hultborn R, Svensson J, Henrikson O, Mölne J, et al. Heterogeneity of microsphere distribution in resected liver and tumour tissue following selective intrahepatic radiotherapy. EJNMMI Res. 2014;4.
Högberg J, Rizell M, Hultborn R, Svensson J, Henrikson O, Mölne J, et al. Increased absorbed liver dose in selective internal radiation therapy (SIRT) correlates with increased sphere-cluster frequency and absorbed dose inhomogeneity. EJNMMI Phys. 2015;2:1–17.
Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–63.
Schiro BJ, Amour ES, Harnain C, Gandhi RT. Management of high hepatopulmonary shunts in the setting of Y90 radioembolization. Tech Vasc Interv Radiol. 2019;22:58–62.
Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4.
Van Roekel C, Van Den Hoven AF, Bastiaannet R, Bruijnen RCG, Braat AJAT, De Keizer B, et al. Use of an anti-reflux catheter to improve tumor targeting for holmium-166 radioembolization-a prospective, within-patient randomized study. Eur J Nucl Med Mol Imaging. 2021;48:1658–68.
Reinders MTM, van Erpecum KJ, Smits MLJ, Braat AJAT, de Bruijne J, Bruijnen R, et al. Safety and efficacy of 166Ho radioembolization in hepatocellular carcinoma: the HEPAR Primary Study. J Nucl Med. 2022;63:1891–8.
van Roekel C, Harlianto NI, Braat AJAT, Prince JF, van den Hoven AF, Bruijnen RCG, et al. Evaluation of the safety and feasibility of same-day holmium-166-radioembolization simulation and treatment of hepatic metastases. J Vasc Interv Radiol. 2020;31:1593–9.
Aramburu J, Antón R, Rivas A, Ramos JC, Sangro B, Bilbao JI. Computational assessment of the effects of the catheter type on particle–hemodynamics during liver radioembolization. J Biomech. 2016;49:3705–13.
Lertxundi U, Aramburu J, Rodríguez-Fraile M, Sangro B, Antón R. Computational study of the microsphere concentration in blood during radioembolization. Mathematics. 2022;10:4280.
Aramburu J, Antón R, Rivas A, Ramos JC, Sangro B, Bilbao JI. The role of angled-tip microcatheter and microsphere injection velocity in liver radioembolization: a computational particle–hemodynamics study. Int J Numer Method Biomed Eng. 2017;33:e2895.
Taebi A, Janibek N, Goldman R, Pillai R, Vu CT, Roncali E. The impact of injection distance to bifurcations on yttrium-90 distribution in liver cancer radioembolization. J Vasc Interv Radiol. 2022;33:668–77.
Bomberna T, Koudehi GA, Claerebout C, Verslype C, Maleux G, Debbaut C. Transarterial drug delivery for liver cancer: numerical simulations and experimental validation of particle distribution in patient-specific livers. Expert Opin Drug Deliv. 2021;18:409–22.
Acknowledgements
The authors would like to thank Camilo M. Correa-Alfonso for his helpful comments during the conception and development of this project.
Funding
This work was supported by the Loeffler Team Science Seed Funding Program granted by the Department of Radiation Oncology of the Massachusetts General Hospital.
Author information
Authors and Affiliations
Contributions
C.H.-B., E.W.-K., and A.B. were equally involved in the conceptualization and design of the study. J.D.W., R.J.D., C.B., H.P., and W.B. were responsible for the development of adult liver computational phantoms. C.H.-B. developed the stochastic model for liver radioembolization, under the supervision of E.W.-K. and A.B. All authors were involved in data analysis and interpretation. C.H.-B. wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Eric Wehrenberg-Klee reports consulting fees from SIRTEX, Embolx, Avenge Biosciences, and Cytosite Bio. He has served on advisory boards for Delcath and Eisai. He is an IDMC member for Replimune. He receives clinical research funding from Boston Scientific. He is grant funded by NCI K08-245257.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huesa-Berral, C., Withrow, J.D., Dawson, R.J. et al. MIDOS: a novel stochastic model towards a treatment planning system for microsphere dosimetry in liver tumors. Eur J Nucl Med Mol Imaging 51, 1506–1515 (2024). https://doi.org/10.1007/s00259-023-06567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06567-9