Abstract
Purpose
Depositions of tau fibrils are implicated in diverse neurodegenerative disorders, including Alzheimer’s disease, and precise assessments of tau pathologies and their impacts on neuronal survival are crucial for pursuing the neurodegenerative tau pathogenesis with and without potential therapies. We aimed to establish an in vivo imaging system to quantify tau accumulations with positron emission tomography (PET) and brain atrophy with volumetric MRI in rTg4510 transgenic mice modeling neurodegenerative tauopathies.
Methods
A total of 91 rTg4510 and non-transgenic control mice underwent PET with a tau radiotracer, 18F-PM-PBB3, and MRI at various ages (1.8–12.3 months). Using the cerebellum as reference, the radiotracer binding in target regions was estimated as standardized uptake value ratio (SUVR) and distribution volume ratio (DVR). Histopathological staining of brain sections derived from scanned animals was also conducted to investigate the imaging-neuropathology correlations.
Results
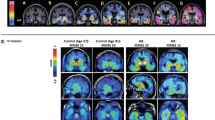
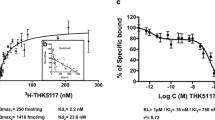
18F-PM-PBB3 SUVR at 40–60 min in the neocortex, hippocampus, and striatum of rTg4510 mice agreed with DVR, became significantly different from control values around 4–5 months of age, and progressively and negatively correlated with age and local volumes, respectively. Neocortical SUVR also correlated with the abundance of tau inclusions labeled with PM-PBB3 fluorescence, Gallyas-Braak silver impregnation, and anti-phospho-tau antibodies in postmortem assays. The in vivo and ex vivo 18F-PM-PBB3 binding was blocked by non-radioactive PM-PBB3. 18F-PM-PBB3 yielded a 1.6-fold greater dynamic range for tau imaging than its ancestor, 11C-PBB3.
Conclusion
Our imaging platform has enabled the quantification of tau depositions and consequent neuronal loss and is potentially applicable to the evaluation of candidate anti-tau and neuroprotective drugs.





Similar content being viewed by others
Data availability
PM-PBB3 and its radiolabeling precursor will be made available upon request. As we granted license on PM-PBB3 and related materials to APRINOIA Therapeutics Inc., material transfer will be subjected to a sublicense agreement with this company if there is potential for commercial application.
Code availability
The datasets supporting the current study have not been deposited in a public repository but are available from the lead contact upon reasonable request.
References
Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59. https://doi.org/10.1146/annurev.neuro.24.1.1121.
Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochem Biophys Acta. 2005;1739:240–50. https://doi.org/10.1016/j.bbadis.2004.08.007.
Myers A, McGonigle P. Overview of Transgenic mouse models for Alzheimer’s disease. Curr Protoc Neurosci. 2019;89:e81. https://doi.org/10.1002/cpns.81.
Imbimbo BP, Ippati S, Watling M, Balducci C. A critical appraisal of tau-targeting therapies for primary and secondary tauopathies. Alzheimer’s Dement : J Alzheimer’s Assoc. 2021. https://doi.org/10.1002/alz.12453.
Panza F, Lozupone M, Seripa D, Daniele A, Watling M, Giannelli G, et al. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat Rev Neurol. 2020;16:213–28. https://doi.org/10.1038/s41582-020-0330-x.
Li C, Götz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discovery. 2017;16:863–83. https://doi.org/10.1038/nrd.2017.155.
Appleby BS, Nacopoulos D, Milano N, Zhong K, Cummings JL. A review: treatment of Alzheimer’s disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1–22. https://doi.org/10.1159/000345791.
Sato H, Takado Y, Toyoda S, Tsukamoto-Yasui M, Minatohara K, Takuwa H, et al. Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci Adv. 2021;7:eab5046. https://doi.org/10.1126/sciadv.abd5046.
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science (New York, NY). 2005;309:476–81. https://doi.org/10.1126/science.1113694.
Shelton LB, Baker JD, Zheng D, Sullivan LE, Solanki PK, Webster JM, et al. Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc Natl Acad Sci USA. 2017;114:9707–12. https://doi.org/10.1073/pnas.1707039114.
DeVos SL, Corjuc BT, Commins C, Dujardin S, Bannon RN, Corjuc D, et al. Tau reduction in the presence of amyloid-β prevents tau pathology and neuronal death in vivo. Brain : J Neurol. 2018;141:2194–212. https://doi.org/10.1093/brain/awy117.
Schaler AW, Runyan AM, Clelland CL, Sydney EJ, Fowler SL, Figueroa HY, et al. PAC1 receptor-mediated clearance of tau in postsynaptic compartments attenuates tau pathology in mouse brain. Sci Transl Med 2021;13. https://doi.org/10.1126/scitranslmed.aba7394
Ishikawa A, Tokunaga M, Maeda J, Minamihisamatsu T, Shimojo M, Takuwa H, et al. In vivo visualization of tau accumulation, microglial activation, and brain atrophy in a mouse model of tauopathy rTg4510. J Alzheimer’s Dis : JAD. 2018;61:1037–52. https://doi.org/10.3233/jad-170509.
Higuchi M, Maeda J, Ji B, Maruyama M, Okauchi T, Tokunaga M, et al. In-vivo visualization of key molecular processes involved in Alzheimer’s disease pathogenesis: insights from neuroimaging research in humans and rodent models. Biochem Biophys Acta. 2010;1802:373–88. https://doi.org/10.1016/j.bbadis.2010.01.003.
Colato E, Chiotis K, Ferreira D, Mazrina MS, Lemoine L, Mohanty R, et al. Assessment of tau pathology as measured by 18F-THK5317 and 18F-flortaucipir PET and their relation to brain atrophy and cognition in Alzheimer’s disease. J Alzheimer’s Dis : JAD. 2021;84:103–17. https://doi.org/10.3233/jad-210614.
Berron D, Vogel JW, Insel PS, Pereira JB, **e L, Wisse LEM, et al. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain : J Neurol. 2021;144:2771–83. https://doi.org/10.1093/brain/awab114.
Shimada H, Kitamura S, Shinotoh H, Endo H, Niwa F, Hirano S, et al. Association between Aβ and tau accumulations and their influence on clinical features in aging and Alzheimer’s disease spectrum brains: A [(11)C]PBB3-PET study. Alzheimer’s Dement (Amsterdam, Netherlands). 2017;6:11–20. https://doi.org/10.1016/j.dadm.2016.12.009.
Tagai K, Ono M, Kubota M, Kitamura S, Takahata K, Seki C, et al. High-Contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron. 2021;109:42-58.e8. https://doi.org/10.1016/j.neuron.2020.09.042.
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–90. https://doi.org/10.1038/nature23002.
Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B, et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580:283–7. https://doi.org/10.1038/s41586-020-2043-0.
Mishra SK, Yamaguchi Y, Higuchi M, Sahara N. Pick’s Tau fibril shows multiple distinct PET probe binding sites: insights from computational modelling. 2020;22. https://doi.org/10.3390/ijms22010349
Brendel M, Jaworska A, Probst F, Overhoff F, Korzhova V, Lindner S, et al. Small-animal PET imaging of tau pathology with 18F-THK5117 in 2 transgenic mouse models. J Nucl Med : Off Publication, Soc Nucl Med. 2016;57:792–8. https://doi.org/10.2967/jnumed.115.163493.
Brendel M, Barthel H, van Eimeren T, Marek K, Beyer L, Song M, et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 2020;77:1408–19. https://doi.org/10.1001/jamaneurol.2020.2526.
Aguero C, Dhaynaut M, Normandin MD, Amaral AC, Guehl NJ, Neelamegam R, et al. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathol Commun. 2019;7:37. https://doi.org/10.1186/s40478-019-0686-6.
Honer M, Gobbi L, Knust H, Kuwabara H, Muri D, Koerner M, et al. Preclinical evaluation of (18)F-RO6958948, (11)C-RO6931643, and (11)C-RO6924963 as novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J Nucl Med : Off Publication, Soc Nucl Med. 2018;59:675–81. https://doi.org/10.2967/jnumed.117.196741.
Chaney AM, Lopez-Picon FR, Serrière S, Wang R, Bochicchio D, Webb SD, et al. Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: a collaborative multi-modal study. Theranostics. 2021;11:6644–67. https://doi.org/10.7150/thno.56059.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108. https://doi.org/10.1016/j.neuron.2013.07.037.
Weng CC, Hsiao IT, Yang QF, Yao CH, Tai CY, Wu MF, et al. Characterization of (18)F-PM-PBB3 ((18)F-APN-1607) uptake in the rTg4510 mouse model of tauopathy. Molecules (Basel, Switzerland). 2020;25. https://doi.org/10.3390/molecules25071750.
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. https://doi.org/10.1016/j.neuron.2007.01.010.
Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14:399–415. https://doi.org/10.1038/s41582-018-0013-z.
Shoeibi A, Olfati N, Litvan I. Preclinical, phase I, and phase II investigational clinical trials for treatment of progressive supranuclear palsy. Expert Opin Investig Drugs. 2018;27:349–61. https://doi.org/10.1080/13543784.2018.1460356.
Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci : Off J Soc Neurosci. 2005;25:10637–47. https://doi.org/10.1523/jneurosci.3279-05.2005.
Sahara N, Ren Y, Ward S, Binder LI, Suhara T, Higuchi M. Tau oligomers as potential targets for early diagnosis of tauopathy. J Alzheimer’s Dis : JAD. 2014;40(Suppl 1):S91–6. https://doi.org/10.3233/jad-132429.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab : Off J Int Soc Cereb Blood Flow Metab. 1996;16:834–40. https://doi.org/10.1097/00004647-199609000-00008.
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab : Off J Int Soc Cereb Blood Flow Metab. 1996;16:42–52. https://doi.org/10.1097/00004647-199601000-00005.
Ono M, Sahara N, Kumata K, Ji B, Ni R, Koga S, et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain : J Neurol. 2017;140:764–80. https://doi.org/10.1093/brain/aww339.
Hashimoto H, Kawamura K, Igarashi N, Takei M, Fujishiro T, Aihara Y, et al. Radiosynthesis, photoisomerization, biodistribution, and metabolite analysis of 11C-PBB3 as a clinically useful PET probe for imaging of tau pathology. J Nucl Med : Off Publication, Soc Nucl Med. 2014;55:1532–8. https://doi.org/10.2967/jnumed.114.139550.
Ni R, Ji B, Ono M, Sahara N, Zhang MR, Aoki I, et al. Comparative in vitro and in vivo quantifications of pathologic tau deposits and their association with neurodegeneration in tauopathy mouse models. J Nucl Med: Off Publication, Soc Nucl Med. 2018;59:960–6. https://doi.org/10.2967/jnumed.117.201632.
Takeuchi H, Imamura K, Ji B, Tsukita K, Enami T, Takao K, et al. Nasal vaccine delivery attenuates brain pathology and cognitive impairment in tauopathy model mice. NPJ Vaccines. 2020;5:28. https://doi.org/10.1038/s41541-020-0172-y.
Hashimoto H, Kawamura K, Takei M, Igarashi N, Fujishiro T, Shiomi S, et al. Identification of a major radiometabolite of [11C]PBB3. Nucl Med Biol. 2015;42:905–10. https://doi.org/10.1016/j.nucmedbio.2015.08.006.
Tai YC, Ruangma A, Rowland D, Siegel S, Newport DF, Chow PL, et al. Performance evaluation of the microPET focus: a third-generation microPET scanner dedicated to animal imaging. J Nucl Med : Off Publication, Soc Nucl Med. 2005;46:455–63.
Kang HG, Tashima H, Nishikido F, Akamatsu G, Wakizaka H, Higuchi M, et al. Initial results of a mouse brain PET insert with a staggered 3-layer DOI detector. Phys Med Biol. 2021;66. https://doi.org/10.1088/1361-6560/ac311c
Li L, Liu FT, Li M, Lu JY, Sun YM, Liang X, et al. Clinical utility of (18) F-APN-1607 tau PET imaging in patients with progressive supranuclear palsy. Mov Disord : Off J Mov Disord Soc. 2021;36:2314–23. https://doi.org/10.1002/mds.28672.
Pluta R, Ułamek-Kozioł M. Tau protein-targeted therapies in Alzheimer’s disease: current state and future perspectives. In: Huang X, editor. Alzheimer’s Disease: Drug Discovery. Brisbane (AU): Exon Publications Copyright: The Authors.; 2020
Acknowledgements
The authors thank Jun Maeda at the National Institutes for Quantum Science and Technology for critical discussions, and Masaki Tokunaga, Nobuhiro Nitta, Sayaka Shibata, Takeharu Minamihisamatsu, Kana Osawa, Shoko Uchida, and Sayuri Sasaki at the National Institutes for Quantum Science and Technology for technical assistance. The authors are grateful to the staff of the Department of Advanced Nuclear Medicine Sciences, Institute for Quantum Medical Science, National Institutes for Quantum Science and Technology, for their technical assistance in radiopharmaceuticals production.
Funding
This study was supported in part by AMED under Grant Number JP19dk0207049, JP22dk0207063, JP21dm0207072, and JP21dk0207046, by JST Grant Number JPMJCR1652 and JPMJMS2024, and by MEXT/JSPS KAKENHI Grant Number JP16H05324, JP19K08005, JP17J06398, and JP17K07113.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Taeko Kimura, Maiko Ono, Chie Seki, Kazuaki Sampei, Masafumi Shimojo, and Yuhei Takado. The first draft of the manuscript was written by Taeko Kimura, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Protocols for the present animal experiments were approved by the Animal Ethics Committees of the National Institutes for Quantum Science and Technology (approval number: 07-1049-31).
Conflict of interest
M.-R.Z. and M.H. hold patents on compounds related to the present report (JP 5422782/EP 12 884 742.3/CA2894994/HK1208672).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimura, T., Ono, M., Seki, C. et al. A quantitative in vivo imaging platform for tracking pathological tau depositions and resultant neuronal death in a mouse model. Eur J Nucl Med Mol Imaging 49, 4298–4311 (2022). https://doi.org/10.1007/s00259-022-05898-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05898-3