Abstract
Plasmids are the primary vectors for intercellular transfer of the oxazolidinone and phenicol cross-resistance gene optrA, while insertion sequences (ISs) are mobile genetic elements that can mobilize plasmid-borne optrA intracellularly. However, little is known about how the IS-mediated intracellular mobility facilitates the dissemination of the optrA gene between plasmid categories that vary in transfer abilities, including non-mobilizable, mobilizable, and conjugative plasmids. Here, we performed a holistic genomic study of 52 optrA-carrying plasmids obtained from searches guided by the Comprehensive Antibiotic Resistance Database. Among the 132 ISs identified within 10 kbp from the optrA gene in the plasmids, IS6 family genes were the most prevalent (86/132). Homologous gene arrays containing IS6 family genes were shared between different plasmids, especially between mobilizable and conjugative plasmids. All these indicated the central role of IS6 family genes in disseminating plasmid-borne optrA. Thirty-three of the 52 plasmids were harbored by Enterococcus faecalis found mainly in humans and animals. By Nanopore sequencing and inverse PCR, the potential of the enterococcal optrA to be transmitted from a mobilizable plasmid to a conjugative plasmid mediated by IS6 family genes was further confirmed in Enterococcus faecalis strains recovered from the effluents of anaerobic digestion systems for treating chicken manure. Our findings highlight the increased intercellular transfer abilities and dissemination risk of plasmid-borne optrA gene caused by IS-mediated intracellular mobility, and underscore the importance of routinely monitoring the dynamic genetic contexts of clinically important antibiotic resistance genes to effectively control this critical public health threat.
Key points
• IS6 was prevalent in optrA-plasmids varying in intercellular transfer abilities.
• Enterococcal optrA-plasmids were widespread among human, animal, and the environment.
• IS6 elevated the dissemination risk of enterococcal optrA-plasmids.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linezolid is a last-resort oxazolidinone antibiotic approved for use in humans to treat serious infections caused by Gram-positive organisms, including vancomycin-resistant enterococci (VRE) (Bozdogan and Appelbaum 2004). Florfenicol is a phenicol antibiotic used exclusively for the prevention and treatment of disease in animals (El Garch et al. 2016; Zhao et al. https://cge.food.dtu.dk/services/MLST/).
Conjugation experiment and stability assay
To test the transferability of optrA-carrying plasmids, filter mating and broth mating experiments were conducted using florfenicol-resistant E. faecalis strains M9 and T30 as donors and rifampicin- and fusidic acid–resistant E. faecalis JH2-2 as the recipient. Mueller-Hinton Agar (MHA) supplemented with 50 mg/L rifampicin, 25 mg/L fusidic acid, and 16 mg/L florfenicol was used for screening enterococcal transconjugants. The presence of optrA in transconjugants was validated by PCR and Sanger sequencing. Conjugation frequency was determined by normalizing the colony formation units (CFUs) of transconjugants to those of recipients (Fioriti et al. 2021). In addition, Staphylococcus aureus RN4220 was employed as a recipient to test the transferability of optrA-carrying plasmids across phylogenetically distant bacteria (Text S1). Natural transformation experiment was conducted to determine the contribution of transformation to the acquisition of optrA-carrying plasmids during conjugation experiments (Text S2). To assess the hereditary stability of optrA-carrying plasmid pEFM9-1, 30-day passages of donors and transconjugants were conducted in Brain Heart Infusion (BHI) broth with or without 16 mg/L florfenicol. Stability was evaluated by comparing resistance rates between day 1 and day 30, which was calculated by normalizing the CFUs on florfenicol-supplemented plates to those on florfenicol-free plates.
Antimicrobial susceptibility testing and PCR analysis
Using E. faecalis ATCC29212 as a quality control strain, antimicrobial susceptibility testing (AST) was performed to test antimicrobial phenotypes of the donor, recipient, and transconjugants. Broth microdilution was conducted following the guidelines provided by the Clinical and Laboratory Standard Institute (CLSI) (CLSI 2018). Quantitative PCR was performed to determine the abundance of the optrA gene in anaerobic effluents. For conventional and inverse PCR, DNA was extracted from pure cultures using Magen HiPure Bacterial DNA Kit (Magen, China). Conventional PCR was performed to test the presence of the optrA gene in all Enterococcus strains. The excision of TUs from the optrA-carrying plasmid pEFM9-1 and pEFT30-1 was tested using inverse PCR. All the primers are given in Table S1. Gel electrophoresis and Sanger sequencing (Sangon Biotech Co., Ltd., Shanghai) of PCR products were performed to confirm the positive amplifications.
Data availability
The Illumina-generated WGS of 50 Enterococcus strain can be accessed by BioProject PRJNA896765 in GenBank. The complete genomes of optrA-carrying E. faecalis strains M9, M61 and T30 were available under BioProject PRJNA846573 in GenBank. The complete sequences of the plasmids pEFM9-1 and pEFT30-1 can be accessed via accession numbers CP098744.1 and CP113829.1, respectively. Raw reads and assembled sequences of the inverse PCR products are available at https://doi.org/10.6084/m9.figshare.21638606.
Results
Characterization of optrA-carrying plasmids obtained from CARD-guided searches
CARD is a curated database specifically for ARGs and their genomic locations and provides timely updated links to relevant genomes uploaded to the NCBI RefSeq database (Alcock et al. 2020; Alcock et al. 2023). Following the workflow described in Fig. 1, a total of 52 non-redundant optrA-carrying plasmids were retrieved from CARD-guided searches. These plasmids, including two novel ones identified in this study from anaerobic digestion systems, originated from nine different countries and range in size from 25 to 246 kbp (Table S2). The host bacteria of the optrA gene comprised five bacterial species that belong to the phylum Firmicutes, including Clostridium perfringens, Enterococcus faecalis, Enterococcus faecium, Enterococcus hirae, and Lactococcus garvieae (Fig. 2a, Table S2). Among these, E. faecalis was the most dominant host bacteria (33/52) and was found to be widely distributed in animal-, human-, and environment-related settings, as well as in pet food (Fig. 2a, Table S2). According to plasmid classification, the 52 plasmids were categorized into 24 non-mobilizable, 19 mobilizable, and 9 conjugative plasmids. E. faecalis and E. faecium were found to harbor all three categories of plasmids, with mobilizable (14/19) and conjugative (7/9) plasmids being predominantly detected in E. faecalis (Fig. 2b). Detailed information on the plasmids is provided in Table S2. A total of 132 IS genes, aligned to 17 specific ISs from 8 IS families, were identified within 10 kbp upstream and downstream from the optrA gene (Fig. 2c, Fig. S2). Among them, 86 ISs belonging to the IS6 family, notably IS1216E (63/132) and ISEnfa1 (17/132), were the most prevalent in the vicinity of the optrA gene in all three categories of plasmids, particularly in the mobilizable and conjugative plasmids (Fig. 2c, Fig. S2a). It was also shown that the IS6 family was predominantly carried by E. faecalis (47/63) (Fig. S2b).
Profiles of the 52 non-redundant optrA-carrying plasmids. a Distribution of host bacteria isolated from animal-, human- and environment-related settings, as well as pet food. b Distribution of non-mobilizable, mobilizable, and conjugative optrA-carrying plasmids in different host bacteria. c Distribution of insertion sequence (IS) families within 10 kbp from the optrA gene in different categories of plasmids. Distribution patterns of specific ISs are shown in Fig. S2
The optrA-containing segments (~ 20 kbp) from the 52 plasmids were subjected to phylogenetic analysis using the Neighbor-Joining method. For the genomic comparison, the order of optrA-containing segments corresponded to that of the branches in phylogenetic tree. It was found that the IS6 family sequences were highly prevalent in three distinct clades of the phylogenetic tree (Fig. 3). In the three clades, the overlapped genomic contexts either contained or were bracketed by IS6 family sequences (Fig. 3). This suggested that IS6 family sequences are likely to mediate the genetic exchange of the optrA-containing segments among plasmids within these respective clades. Clade 1 consisted of two segments from mobilizable plasmids originating from animals. Clade 2 contained three segments from mobilizable plasmids with origins in animals, the environment, and pet food. Finally, Clade 3 included five segments from conjugative, one segment from mobilizable, and three segments from non-mobilizable plasmids with origins in animals, the environment, and pet food. It is noteworthy that IS6 family sequences were also found to be prevalent in several other clades. Nevertheless, the majority of these IS6 family sequences were situated outside of the overlapped regions. While these IS6 family sequences might have contributed to the dissemination of optrA-containing segments, they might not be involved in the most recent transmission events.
Phylogenetic analysis and genomic comparison of DNA segments from 52 optrA-carrying plasmids. DNA segments from 10 kbp upstream to 10 kbp downstream of the optrA gene were selected. Segment sequences were aligned using MAFFT with FFT-NS-2 strategy. A Neighbor-Joining tree was constructed based on the 20-kbp plasmid segments, with a bootstrap value of 1000. The relative support from 1000 replications was indicated by the numbers next to the branching points, with values > 60 shown in the figure. The segments were annotated using Prokka. Genomic comparison was performed using Easyfig, with arrows indicating the orientations of genes. Details of the 52 optrA-carrying plasmids and selected segments are provided in Table S2
The optrA gene and its enterococcal hosts in anaerobic digestion effluents
The database analysis revealed that IS6 family genes were predominant in optrA-carrying plasmids, with E. faecalis being the dominant host primarily distributed in clinical and animal-related settings. However, limited knowledge exists regarding the dissemination of the optrA gene carried by Enterococcus strains in the anaerobic digestion systems for treating animal manure. In order to further understand the role of IS6 family genes in the dissemination of the enterococcal optrA gene among plasmids with different transfer abilities, a total of 156 Enterococcus strains were isolated from the mesophilic (37 °C) and thermophilic (55 °C) effluents of anaerobic digestion systems for treating chicken manure. No significant difference in absolute and relative abundances of the optrA gene was observed between mesophilic and thermophilic effluents (P > 0.05) (Fig. S3a). PCR assays indicated that 54 out of 75 isolated Enterococcus strains in the mesophilic effluent, and 46 out of 81 isolated Enterococcus strains in the thermophilic effluent carried the optrA gene, respectively (Fig. S3b). Among the isolated strains, a total of 50 strains, with 25 from mesophilic effluent and 25 from thermophilic effluent, were randomly selected for whole-genome sequencing (WGS) using Illumina short-read sequencing technology. WGS-based annotations identified one E. faecium and 49 Enterococcus faecalis, assigned to nine different sequence types (STs) by multi-locus sequence ty** (MLST) (Table S3). All the optrA-carrying strains were assigned to E. faecalis ST368, ST631, and ST81, and it was found that strains assigned to these three STs all carried the optrA gene (Fig. S3c, Table S3). Three optrA-carrying strains, namely, E. faecalis M9, M61, and T30, were selected as representative strains of the three optrA-carrying STs, i.e., ST368, ST631, and ST81, respectively. These strains were subjected to Nanopore long-read sequencing to obtain complete genomes. Complete sequences revealed that optrA is located in a 66,643-bp plasmid (designated as “pEFM9-1”) of E. faecalis ST368 strain M9, a 65374-bp plasmid (designated as “pEFT30-1”) of E. faecalis ST81 strain T30, and the chromosome of E. faecalis ST631 strain M61. Illumina-generated sequences of all the 50 E. faecalis strains were further mapped against the two optrA-carrying plasmids (Fig. S4). Map** results indicated that pEFM9-1 was carried by all the E. faecalis ST368 strains (Fig. S4a and b), while pEFT30-1 was carried by the E. faecalis ST81 strain (Fig. S4c and d).
Characterization of enterococcal optrA-carrying plasmids in anaerobic digestion systems treating animal manure
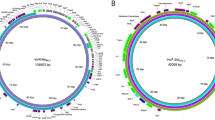
Two novel optrA-carrying plasmids were detected in E. faecalis ST368 and ST81 strains, designated as pEFM9-1 and pEFT30-1, respectively (Fig. S4). Plasmid pEFT30-1 encodes all four components, including oriT, relaxase, T4CP, and T4SS, and thus is categorized as a self-transmissible “conjugative plasmid” (Fig. 4a) (Che et al. 2021; Smillie et al. 2010). On the other hand, pEFM9-1 encodes only oriT and relaxase, and is categorized as a “mobilizable plasmid” (Fig. 4a) (Che et al. 2021; Smillie et al. 2010). We further predicted conjugation components within the genomes of E. faecalis M9 strain using oriTfinder. As a result, two additional plasmids were found to harbor T4CP (Table S4), which could potentially facilitate the conjugation transfer of the optrA-carrying plasmid pEFM9-1. It is worth noting, however, that no T4SS was found within the genomes. This absence might be attributed to the possibility that potential T4SS elements were not included in the oriTfinder database. Both filter and broth mating experiments confirmed that plasmids pEFT30-1 and pEFM9-1 can be transferred to the recipient strain E. faecalis JH2-2 through conjugation. The transconjugants of both plasmids showed elevated MICs of respective antibiotics compared to the recipient strain (Table S5). No transconjugants were obtained using S. aureus RN4220 as the recipient, indicating the two optrA-carrying plasmids are unable to transfer to S. aureus by conjugation. No transformants were obtained using E. faecalis JH2-2 as recipient under natural cultivation conditions, suggesting the transformation may have negligible contribution to the intraspecies conjugation frequency. The intraspecies conjugation frequency of pEFT30-1 was approximately 20 times that of pEFM9-1 on filter and 14 times that of pEFM9-1 in broth (Fig. 4b).
Enterococcal optrA-carrying plasmids from effluents of mesophilic (37 °C) and thermophilic (55 °C) digestion systems for treating chicken manure. a Circular maps of optrA-carrying plasmids pEFM9-1 and pEFT30-1. Antibiotic resistance genes (in red), insertion sequences (in green), and plasmid conjugation components (in yellow) were predicted by CARD RGI, Prokka, and oriTfinder, respectively. Complete sequences were obtained by performing hybrid assembly of Nanopore and Illumina sequences. b Details of the two optrA-carrying plasmids. Conjugation experiments were performed both on filter and in broth, using E. faecalis JH2-2 as the recipient cell
Although the intraspecies conjugation frequency of the mobilizable plasmid pEFM9-1 is relatively lower than that of pEFT30-1, it harbors more diverse ARGs conferring resistance to a wider spectrum of antibiotics (Fig. 4, Table S5). A 30-day stability test indicated that pEFM9-1 could be stably inherited by the offspring of the donor and transconjugant in broth culture, regardless of whether florfenicol was supplemented (Fig. S5). Additionally, pEFM9-1 is a mosaic plasmid embedded with multiple IS6 family genes, including six copies of IS1216E, two copies of ISEnfa1, and two copies of IS1297.
Excision of circularizable structure flanked by IS6 family genes in pEFM9-1 and pEFT30-1
Inverse PCR primers were designed to target three segments of pEFM9-1 and one segment in pEFT30-1 (Table S1) in the current study. The formation of the four tested TUs was confirmed by Sanger sequencing of PCR products. As shown in the schematic diagram (Fig. S6a), “segment 1” containing bcr genes was circularized through recombination of two copies of ISEnfa1, while segments 2 and 3 containing optrA and multiple ARGs were circularized through recombination of two copies of IS1216E. The TU in pEFT30-1 containing optrA was formed by recombination of two copies of ISEnfa1 (Fig. S6b). Gel images of inverse PCR products are shown in Fig. S6c.
An unnamed plasmid (accession number: LR962139.1) was retrieved by searching the sequence of pEFT30-1 against the NCBI nucleotide database. The unnamed plasmid showed perfect matching with pEFT30-1, except for a segment in the middle (Fig. 5a). This unmatched segment, which contained optrA and was flanked by two copies of ISEnfa1 in pEFT30-1, was designated as the “acquired segment” (Fig. 5a). In addition, the “acquired segment” containing bcr genes and the optrA gene showed high homology with the circularizable segments 1 and 2 in pEFM9-1 (Fig. 5b), with more than 90% identity match.
Genetic contexts of optrA-carrying plasmids pEFM9-1 (categorized as “mobilizable plasmid”) and pEFT30-1 (categorized as “conjugative plasmid”). a Comparison between the complete sequences of pEFT30-1 and a plasmid deposited in the NCBI nucleotide database (accession number: LR962139.1). Plasmid pEFT30-1 shows high identity to the other plasmid, except for a unmatched segment (in the open rectangle, designated as “acquired segment”) flanked by two ISEnfa1. b Comparison of the sequence segments of pEFM9-1 and pEFT30-1. Arrows in red, green, and blue indicate the position and orientation of antibiotic resistance genes, mobile elements, and other genes, respectively. Folded arrows indicate the starting position and orientation of primers for inverse PCR. Note that the sizes of translocatable units were calculated assuming a common case: Only homologous recombination of paired IS6 family genes occurred. c A putative illustration for the IS6 family-mediated intracellular mobility and plasmid-mediated intercellular transfer of the optrA gene. Same-colored rounded rectangles represent the same cell. Processes validated by inverse PCR are indicated by solid black arrows, while putative processes are indicated by dotted arrows. Circles in orange represent the “conjugative plasmid,” while those in gray represent the “mobilizable plasmid”
Putative IS-mediated interaction between plasmids pEFM9-1 and pEFT30-1
Based on the findings, a hypothetical process of IS6 family-mediated interactions of plasmids was proposed (Fig. 5c): An ancestral plasmid, similar to pEFT30-1 but lacking the optrA-containing segment, was likely capable of autonomous transfer between bacteria due to its complete conjugation components. Once this plasmid invaded an enterococcal cell that already harbored an optrA-carrying plasmid containing multiple IS6 family genes, such as pEFM9-1, the optrA-carrying plasmid could release TUs through recombination events mediated by ISEnfa1 or IS1216E. Subsequently, these TUs could integrate into the conjugative plasmid, leading to the acquisition of the optrA gene by the conjugative plasmid. The optrA gene located in the “acquired segment” of pEFT30-1 was also found to be mobilized by the two ISEnfa1 genes in the same orientation, which facilitated further mobility of the optrA gene (Fig. S6b, Fig. 5c).
It should be noted that a small unmatched segment exists between the “acquired segment” and “segment 2” (Fig. 5b), suggesting that the acquisition of the optrA gene in pEFT30-1 may involve unknown intermediate processes. In addition, as experimental evidence supporting IS6-mediated integration of the optrA-carrying TU into the ancestral plasmid of pEFT30-1 is currently lacking, the interaction between pEFM9-1 and pEFT30-1 remains a hypothetical and potential event. However, considering that both pEFM9-1 and pEFT30-1 were detected from E. faecalis ST368 and ST81, respectively, in the same sample (i.e., the thermophilic effluent), and that no perfect match of the “acquired segment” of pEFT30-1 was found in the NCBI database (as of Dec. 22, 2022), the genetic exchange between pEFM9-1 and pEFT30-1 remains the most plausible hypothesis.
Genomic comparison of circularizable structure flanked by IS6 family genes
To further investigate the prevalence of IS6 family-flanked segments, we searched the circularizable segments against the NCBI nucleotide database. To ensure representativeness, we selected only non-redundant sequences from top hits on the NCBI website as of Dec. 22, 2022. These sequences were required to share high identity with the reference sequence and are present in different host bacteria or genomic types. The results are visualized in Fig. S7. Sequences similar to “segment 1” (~ 100% coverage and identity) were identified in plasmids and chromosomes of diverse host bacteria, including Enterococcus, Streptococcus, and Jeotgalibaca, which were isolated from human-, animal-, and food-related samples in different countries (Fig. S7a). On the other hand, sequences perfectly matching “segment 2” were detected in plasmids of manure-borne and food-borne E. faecalis, with a notable finding of a “segment 2”-like sequence in the chromosome of Fusobacterium hominis of human origin (Fig. S7b). No highly identical matches to the “segment 3” of pEFM9-1 and the “acquired segment” of pEFT30-1 were found in the NCBI nucleotide database as of December 22, 2022.
Discussion
It has been well-established that conjugative plasmids play a key role in intercellular transfer of ARGs independently (Coluzzi et al. 2022; Smillie et al. 2010), making optrA gene located on conjugative plasmids of particular concern for dissemination. In contrast, non-mobilizable and mobilizable plasmids either cannot achieve cell-to-cell conjugation or require assistance from other DNA molecules, resulting in their potential to disseminate ARGs being often overlooked or underestimated. Interestingly, our study revealed that DNA segments containing the optrA gene with similar sequences were distributed among all three categories of plasmids, and these segments showed close phylogenetic relationships. In-depth analysis of overlap** regions of optrA-containing segments reveals close association between optrA and ISs. Key transfer units, specifically ISs-optrA, are conserved across various plasmid categories. Notably, this close association with ISs has significantly expanded the spread of plasmid-borne optrA across diverse ecological niches. For instance, IS6-optrA has been observed in isolates from animal, environmental, and human settings (Fig. 3). This implies that ISs may play a pivotal role in facilitating the genetic exchange of optrA-containing segments, thereby driving the dynamic evolution and dissemination of optrA-carrying plasmids among bacteria through a One Health approach.
Furthermore, it was found that most of the gene arrays shared by the optrA-containing segments, which contributed to the clustering of phylogenetic tree branches, contained or were bracketed by various ISs (Fig. 3). Among these, the IS6 family genes were prevalent in all three categories of plasmids, and were predominant in mobilizable and conjugative plasmids (Figs. 2 and 3, and Fig. S2). This suggested that IS6 family genes played a central role in mobilizing the optrA gene among plasmids. Under the mediation of ISs, the dissemination risk of the optrA gene may be amplified when being transmitted to plasmids with stronger transfer abilities. Once integrated into a conjugative plasmid, the optrA gene may spread between different strains, species, or even genera of bacteria (Schwarz et al. 2021). Therefore, although the intercellular transfer ability of non-mobilizable and mobilizable plasmids is limited, their role in disseminating optrA and other resistance genes cannot be ignored. It was also found that the predominant host bacteria for the optrA-carrying plasmids were E. faecalis, which were isolated not only from humans and animals but also from the environment (Fig. 2, Table S2). This suggested that E. faecalis could colonize diverse habitats, making it the super carrier and spreader of the optrA-carrying plasmids. Therefore, we hypothesize that the optrA-carrying plasmids harbored by E. faecalis can survive animal manure treatments, and IS-mediated interactions between plasmids may increase the dissemination risk of optrA when being discharged into the environment.
Anaerobic digestion is one of the well-established techniques for treating animal manures (Khoshnevisan et al. 2021). However, in the current study, even after anaerobic digestion with HRT of 20 days, an optrA-carrying plasmid, designated as “pEFM9-1” and categorized as a mobilizable plasmid, was detected in all the E. faecalis ST368 strains that survived in the mesophilic and thermophilic effluents (Fig. S4a and b), indicating that pEFM9-1 could be stably inherited by the offspring. The heredity stability of the plasmid pEFM9-1 was further confirmed by the 30-day passage experiment (Fig. S5) and could be genetically explained by the presence of a toxin-antitoxin (TA) system in pEFM9-1 (Fig. 4a), which is a DNA module responsible for plasmid maintenance (Partridge et al. 2018; Van Melderen and Saavedra De Bast 2009; Wein and Dagan 2020). The TA system sets a stable stage for the optrA-carrying plasmid to interact with other DNA molecules in the host cell, favoring the dissemination of optrA even under harsh anaerobic digestion conditions. At the same time, another optrA-carrying plasmid, designated as “pEFT30-1,” was detected in E. faecalis ST81 from the thermophilic effluent. The pEFT30-1 was categorized as a self-transmissible conjugative plasmid, with conjugation frequency approximately 14–20 times that of the mobilizable plasmid pEFM9-1 (Fig. 4b). This indicated that pEFT30-1 was more efficient in horizontally transferring the optrA gene compared to pEFM9-1.
Interestingly, interactions between pEFM9-1 and pEFT30-1 mediated by IS6 family genes might have occurred, which was inferred based on at least three hints. Firstly, an unnamed plasmid deposited in NCBI showed a perfect match with pEFT30-1, except for an optrA-containing segment flanked by ISEnfa1 (arranged as ISEnfa1-uppP-bcrBAR-IS1216E-fexA-optrA-ermA-ISEnfa1) (Fig. 5a). This suggested that pEFT30-1 and the unnamed plasmid shared a common ancestral plasmid, and the ISEnfa1-flanked optrA-containing segment was likely acquired, hence referred to as the “acquired segment.” Secondly, the ISEnfa1-uppP-bcrBAR-ISEnfa1 segment was found to be present in various host bacteria from different origins (Fig. S7a). This suggested that the segment was relatively conserved, and thus, the arrangement of the “acquired segment” in pEFT30-1 was likely formed by inserting an IS1216E-fexA-optrA-ermA segment into the ISEnfa1-uppP-bcrBAR-ISEnfa1 segment (Fig. 5b). Thirdly, the ISEnfa1-uppP-bcrBAR-ISEnfa1 segment (segments 1) and the IS1216E-fexA-optrA-ermA-IS1216E-IS1216E segment (segment 2) in pEFM9-1 were confirmed to form TUs by inverse PCR (Fig. S6a and c), and these circularizable segments showed high homology to the “acquired segment” in pEFT30-1 (Fig. 5b). As there were no paired IS1216E genes in the “acquired segment,” the IS1216E-fexA-optrA-ermA segment was unable to excise from pEFT30-1 in principle. Hence, if it occurred, the optrA gene would be transmitted unidirectionally from the mobilizable plasmid pEFM9-1 to the conjugative plasmid pEFT30-1, amplifying the risk of optrA dissemination. Additionally, the optrA gene in the conjugative plasmid pEFM9-1 was also confirmed to be mobilized by recombination of two copies of ISEnfa1 (Fig. S6b and c), which was first reported, enabling further mobility of the optrA gene between genomes. It is worth noting that there is a lack of evidence regarding the direct interaction between optrA-carrying plasmids and the IS6-mediated integration of optrA-carrying TUs. In order to capture the dynamic interactions mediated by ISs in the optrA transfer process, it is imperative to conduct more comprehensive whole-genome screenings across a broader spectrum of isolates in the future study.
In our study, the role of IS6 family genes in amplifying the risk of horizontal transfer of the plasmid-borne optrA gene was highlighted. However, it is noteworthy that the IS6 family genes may also facilitate the genetic exchange of various clinically important ARGs, including but not limited to the optrA gene (Che et al. 2021; Dai et al. 2023; Partridge et al. 2018; Schwarz et al. 2021; Shan et al. 2020). This exchange can occur not only between plasmids but also between other genomic elements such as chromosomes (Fig. S7b) and ICEs (Shang et al. 2019; Yang et al. 2022). This suggests that the IS6 family genes play a significant role in the dissemination of antibiotic resistance genes. Therefore, routine surveillance to capture the dynamic IS-mediated mobility of ARGs is essential for understanding and controlling the dissemination of antibiotic resistance from a One Health perspective.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alcock BP, Huynh W, Chalil R, Smith KW, Raphenya AR, Wlodarski MA, Edalatmand A, Petkau A, Syed SA, Tsang KK, Baker SJC, Dave M, McCarthy MC, Mukiri KM, Nasir JA, Golbon B, Imtiaz H, Jiang X, Kaur K et al (2023) CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res 51(D1):D690–D699. https://doi.org/10.1093/nar/gkac920
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen AV, Cheng AA, Liu S, Min SY, Miroshnichenko A, Tran HK, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B et al (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48(D1):D517–D525. https://doi.org/10.1093/nar/gkz935
Alikhan N-F, Petty NK, Zakour NLB, Beatson SA (2011) BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom 12:402
Almeida LM, Gaca A, Bispo PM, Lebreton F, Saavedra JT, Silva RA, Basilio ID, Zorzi FM, Filsner PH, Moreno AM, Gilmore MS (2020) Coexistence of the oxazolidinone resistance-associated genes cfr and optrA in Enterococcus faecalis from a healthy piglet in Brazil. Front Public Health 8. https://doi.org/10.3389/fpubh.2020.00518
Arias CA, Murray BE (2012) The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10(4):266–278. https://doi.org/10.1038/nrmicro2761
Bennett PM (2008) Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol 153 Suppl 1(Suppl 1):S347–S357. https://doi.org/10.1038/sj.bjp.0707607
Biggel M, Nuesch-Inderbinen M, Jans C, Stevens MJA, Stephan R (2021) Genetic context of optrA and poxtA in florfenicol-resistant enterococci isolated from flowing surface water in Switzerland. Antimicrob Agents Chemother 65:e01083–e01021. https://doi.org/10.1128/aac.01083-21
Botelho J, Schulenburg H (2021) The role of integrative and conjugative elements in antibiotic resistance evolution. Trends Microbiol 29(1):8–18. https://doi.org/10.1016/j.tim.2020.05.011
Bozdogan B, Appelbaum PC (2004) Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 23(2):113–119. https://doi.org/10.1016/j.ijantimicag.2003.11.003
Brenciani A, Morroni G, Schwarz S, Giovanetti E (2022) Oxazolidinones: mechanisms of resistance and mobile genetic elements involved. J Antimicrob Chemother 77(10):2596–2621. https://doi.org/10.1093/jac/dkac263
Cai J, Chen J, Schwarz S, Wang Y, Zhang R (2021) Detection of the plasmid-borne oxazolidinone/phenicol resistance gene optrA in Lactococcus garvieae isolated from faecal samples. Clin Microbiol Infect 27(9):1358–1359. https://doi.org/10.1016/j.cmi.2021.04.027
Ch’ng JH, Chong KKL, Lam LN, Wong JJ, Kline KA (2019) Biofilm-associated infection by enterococci. Nat Rev Microbiol 17(2):82–94. https://doi.org/10.1038/s41579-018-0107-z
Che Y, Yang Y, Xu X, Brinda K, Polz MF, Hanage WP, Zhang T (2021) Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc Natl Acad Sci USA 118(6):e2008731118. https://doi.org/10.1073/pnas.2008731118
CLSI (2018) Performance standards for antimicrobial susceptibility testing. CLSI supplement M100, 28th edn. Clinical and Laboratory Standards Institute, Wayne, PA
Coluzzi C, Garcillan-Barcia MP, de la Cruz F, Rocha EPC (2022) Evolution of plasmid mobility: origin and fate of conjugative and nonconjugative plasmids. Mol Biol Evol 39(6):msac115. https://doi.org/10.1093/molbev/msac115
D’Andrea MM, Antonelli A, Brenciani A, Di Pilato V, Morroni G, Pollini S, Fioriti S, Giovanetti E, Rossolini GM (2019) Characterization of Tn6349, a novel mosaic transposon carrying poxtA, cfr and other resistance determinants, inserted in the chromosome of an ST5-MRSA-II strain of clinical origin. J Antimicrob Chemother 74(10):2870–2875. https://doi.org/10.1093/jac/dkz278
Dai X, Sun J, Zhu B, Lv M, Chen L, Chen L, Wang X, Huang J, Wang L (2023) Various mobile genetic elements involved in the dissemination of the phenicol-oxazolidinone resistance gene optrA in the zoonotic pathogen Streptococcus suis: a nonignorable risk to public health. Microbiol Spectr. https://doi.org/10.1128/spectrum.04875-22
El Garch F, de Jong A, Simjee S, Moyaert H, Klein U, Ludwig C, Marion H, Haag-Diergarten S, Richard-Mazet A, Thomas V, Siegwart E (2016) Monitoring of antimicrobial susceptibility of respiratory tract pathogens isolated from diseased cattle and pigs across Europe, 2009-2012: VetPath results. Vet Microbiol 194:11–22. https://doi.org/10.1016/j.vetmic.2016.04.009
Fan R, Li D, Fessler AT, Wu C, Schwarz S, Wang Y (2017) Distribution of optrA and cfr in florfenicol-resistant Staphylococcus sciuri of pig origin. Vet Microbiol 210:43–48. https://doi.org/10.1016/j.vetmic.2017.07.030
Fan R, Li D, Wang Y, He T, Fessler AT, Schwarz S, Wu C (2016) Presence of the optrA gene in methicillin-resistant Staphylococcus sciuri of porcine origin. Antimicrob Agents Chemother 60(12):7200–7205. https://doi.org/10.1128/aac.01591-16
Fioriti S, Coccitto SN, Cedraro N, Simoni S, Morroni G, Brenciani A, Mangiaterra G, Vignaroli C, Vezzulli L, Biavasco F, Giovanetti E (2021) Linezolid resistance genes in enterococci isolated from sediment and zooplankton in two Italian coastal areas. Appl Environ Microbiol 87(9):e02958–e02920. https://doi.org/10.1128/aem.02958-20
Garcillan-Barcia MP, Francia MV, de la Cruz F (2009) The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev 33(3):657–687. https://doi.org/10.1111/j.1574-6976.2009.00168.x
Grant JR, Enns E, Marinier E, Mandal A, Herman EK, Chen CY, Graham M, Van Domselaar G, Stothard P (2023) Proksee: in-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. https://doi.org/10.1093/nar/gkad326
He T, Shen Y, Schwarz S, Cai J, Lv Y, Li J, Fessler AT, Zhang R, Wu C, Shen J, Wang Y (2016) Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J Antimicrob Chemother 71(6):1466–1473. https://doi.org/10.1093/jac/dkw016
Johnson CM, Grossman AD (2015) Integrative and conjugative elements (ICEs): what they do and how they work. Annu Rev Genet 49:577–601. https://doi.org/10.1146/annurev-genet-112414-055018
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166. https://doi.org/10.1093/bib/bbx108
Khoshnevisan B, Duan N, Tsapekos P, Awasthi MK, Liu Z, Mohammadi A, Angelidaki I, Tsang DCW, Zhang Z, Pan J, Ma L, Aghbashlo M, Tabatabaei M, Liu H (2021) A critical review on livestock manure biorefinery technologies: sustainability, challenges, and future perspectives. Renew Sust Energ Rev 135:110033. https://doi.org/10.1016/j.rser.2020.110033
Larsson DGJ, Flach CF (2022) Antibiotic resistance in the environment. Nat Rev Microbiol 20(5):257–269. https://doi.org/10.1038/s41579-021-00649-x
Li D, Li X-Y, Schwarz S, Yang M, Zhang S-M, Hao W, Du X-D (2019) Tn6674 is a novel enterococcal optrA-carrying multiresistance transposon of the Tn554 family. Antimicrob Agents Chemother 63(9):e00809–e00819. https://doi.org/10.1128/aac.00809-19
Li D, Wang Y, Schwarz S, Cai J, Fan R, Li J, Fessler AT, Zhang R, Wu C, Shen J (2016) Co-location of the oxazolidinone resistance genes optrA and cfr on a multiresistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother 71(6):1474–1478. https://doi.org/10.1093/jac/dkw040
Li R, **e M, Dong N, Lin D, Yang X, Wong MHY, Chan EW, Chen S (2018a) Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. Gigascience 7(3):1–9. https://doi.org/10.1093/gigascience/gix132
Li X, **e Y, Liu M, Tai C, Sun J, Deng Z, Ou HY (2018b) oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res 46(W1):W229–W234. https://doi.org/10.1093/nar/gky352
Liao H, Lu X, Rensing C, Friman VP, Geisen S, Chen Z, Yu Z, Wei Z, Zhou S, Zhu Y (2018) Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge. Environ Sci Technol 52(1):266–276. https://doi.org/10.1021/acs.est.7b04483
Lopatkin AJ, Meredith HR, Srimani JK, Pfeiffer C, Durrett R, You L (2017) Persistence and reversal of plasmid-mediated antibiotic resistance. Nat Commun 8(1):1689. https://doi.org/10.1038/s41467-017-01532-1
Partridge SR, Kwong SM, Firth N, Jensen SO (2018) Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 331(4):e00088–e00017
San Millan A (2018) Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol 26(12):978–985. https://doi.org/10.1016/j.tim.2018.06.007
Schwarz S, Zhang W, Du X-D, Krueger H, Fessler AT, Ma S, Zhu Y, Wu C, Shen J, Wang Y (2021) Mobile oxazolidinone resistance genes in Gram-positive and Gram-negative bacteria. Clin Microbiol Rev 34(3):e00188–e00120. https://doi.org/10.1128/cmr.00188-20
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30(14):2068–2069. https://doi.org/10.1093/bioinformatics/btu153
Shan X, Li X-S, Wang N, Schwarz S, Zhang S-M, Li D, Du X-D (2020) Studies on the role of IS1216E in the formation and dissemination of poxtA-carrying plasmids in an Enterococcus faecium clade A1 isolate. J Antimicrob Chemother 75(11):3126–3130. https://doi.org/10.1093/jac/dkaa325
Shang Y, Li D, Hao W, Schwarz S, Shan X, Liu B, Zhang S-M, Li X-S, Du X-D (2019) A prophage and two ICESa2603-family integrative and conjugative elements (ICEs) carrying optrA in Streptococcus suis. J Antimicrob Chemother 74(10):2876–2879. https://doi.org/10.1093/jac/dkz309
Sharkey LKR, Edwards TA, O'Neill AJ (2016) ABC-F Proteins mediate antibiotic resistance through ribosomal protection. mBio 7(2):e01975–e01915. https://doi.org/10.1128/mBio.01975-15
Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EP, de la Cruz F (2010) Mobility of plasmids. Microbiol Mol Biol Rev 74(3):434–452. https://doi.org/10.1128/MMBR.00020-10
Sullivan MJ, Petty NK, Beatson SA (2011) Easyfig: a genome comparison visualizer. Bioinformatics 27(7):1009–1010. https://doi.org/10.1093/bioinformatics/btr039
Tang B, Wang Y, Luo Y, Zheng X, Qin X, Yang H, Shen Z (2021) Coexistence of optrA and fexA in Campylobacter. Msphere 6(3):e00125–e00121. https://doi.org/10.1128/mSphere.00125-21
Tian T, Qiao W, Han Z, Wen X, Yang M, Zhang Y (2021) Effect of temperature on the persistence of fecal bacteria in ambient anaerobic digestion systems treating swine manure. Sci Total Environ 791:148302. https://doi.org/10.1016/j.scitotenv.2021.148302
Van Melderen L, Saavedra De Bast M (2009) Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet 5(3):e1000437. https://doi.org/10.1371/journal.pgen.1000437
Walsh TR (2018) A one-health approach to antimicrobial resistance. Nat Microbiol 3(8):854–855. https://doi.org/10.1038/s41564-018-0208-5
Wang Y, Li X, Fu Y, Chen Y, Wang Y, Ye D, Wang C, Hu X, Zhou L, Du J, Shen J, **a X (2020) Association of florfenicol residues with the abundance of oxazolidinone resistance genes in livestock manures. J Hazard Mater 399:123059. https://doi.org/10.1016/j.jhazmat.2020.123059
Wang Y, Li X, Wang Y, Schwarz S, Shen J, **a X (2018) Intracellular accumulation of linezolid and florfenicol in optrA-producing Enterococcus faecalis and Staphylococcus aureus. Molecules 23(12). https://doi.org/10.3390/molecules23123195
Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Fessler AT, Wu C, Yu H, Deng X, **a X, Shen J (2015) A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70(8):2182–2190. https://doi.org/10.1093/jac/dkv116
Wein T, Dagan T (2020) Plasmid evolution. Curr Biol 30(19):R1158–R1163. https://doi.org/10.1016/j.cub.2020.07.003
Wick RR, Judd LM, Gorrie CL, Holt KE (2017) Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13(6):e1005595. https://doi.org/10.1371/journal.pcbi.1005595
Wu K, Wang J, Feng H, Li R, Wang X, Yang Z (2022) Complete genome sequence and characterization of Clostridium perfringens type D carrying optrA-plasmid and Tn6218-like transposon. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkac393
Yang X-X, Tian T-T, Qiao W, Tian Z, Yang M, Zhang Y, Li J-Y (2020) Prevalence and characterization of oxazolidinone and phenicol cross-resistance gene optrA in enterococci obtained from anaerobic digestion systems treating swine manure. Environ Pollut 267:115540. https://doi.org/10.1016/j.envpol.2020.115540
Yang Y, Kuang X, Han R-j, Zhai Y-j, He D-d, Zhao J-f, Liu J-h, Hu G-z (2022) Characterization of a novel linezolid eesistance gene optrA and bacitracin resistance locus-carrying multiple antibiotic resistant integrative and conjugative element ICESsu1112S in Streptococccus Suis. Microbiol Spectr 10(1):e01963–e01921
Zhang Y, Walsh TR, Wang Y, Shen J, Yang M (2022) Minimizing risks of antimicrobial resistance development in the environment from a public One Health perspective. China CDC Wkly 4:1105–1109
Zhao Q, Wang Y, Wang S, Wang Z, Du XD, Jiang H, **a X, Shen Z, Ding S, Wu C, Zhou B, Wu Y, Shen J (2016) Prevalence and abundance of florfenicol and linezolid resistance genes in soils adjacent to swine feedlots. Sci Rep 6:32192. https://doi.org/10.1038/srep32192
Zhou Y, Li J, Schwarz S, Zhang S, Tao J, Fan R, Walsh TR, Wu C, Wang Y (2020) Mobile oxazolidinone/phenicol resistance gene optrA in chicken Clostridium perfringens. J Antimicrob Chemother 75(10):3067–3069. https://doi.org/10.1093/jac/dkaa236
Funding
This study was supported by National Natural Science Foundation of China (No. 32141002).
Author information
Authors and Affiliations
Contributions
SL and YZ conceived and designed research. SL and XY conducted experiments. RL and SW contributed new reagents or analytical tools. SL analyzed data. SL wrote the manuscript. YZ, RL, ZH, and MY modified the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This work does not involve any human participants nor live animals performed by any of the listed authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 3.08 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Yang, X., Li, R. et al. IS6 family insertion sequences promote optrA dissemination between plasmids varying in transfer abilities. Appl Microbiol Biotechnol 108, 132 (2024). https://doi.org/10.1007/s00253-023-12858-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12858-w