Abstract
Helicobacter pylori is a highly prevalent human pathogen responsible for chronic inflammation of the gastric tissues, gastroduodenal ulcers, and cancer. The treatment includes a pair of antibiotics with a proton pump inhibitor PPI. Despite the presence of different treatments, the infection rate is still increasing both in developed and develo** states. The challenge of treatment failure is greatly due to the resistance of H. pylori to antibiotics and its side effects. Probiotics potential to cure H. pylori infection is well-documented. Probiotics combined with conventional treatment regime appear to have great potential in eradicating H. pylori infection, therefore, provide an excellent alternative approach to manage H. pylori load and its threatening disease outcome. Notably, anti-H. pylori activity of probiotics is strain specific,therefore establishing standard guidelines regarding the dose and formulation of individual strain is inevitable. This review is focused on probiotic’s antagonism against H. pylori summarizing their three main potential aspects: their efficiency (i) as an alternative to H. pylori eradication treatment, (ii) as an adjunct to H. pylori eradication treatment and (iii) as a vaccine delivery vehicle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
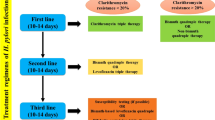
H. pylori resides in more than half of the population on earth (Dunne et al. 2014). They are highly pathogenic when bound to gastric epithelial cells (Hessey et al. 1990). They are Gram negative, helical, flagellated, and microaerophillic organisms, known to cause chronic gastritis, which if uncured eventually may result in duodenal ulcer and gastric cancer (Dunne et al. 2014). H. pylori infections have increased prevalence in develo** states (Moayyedi and Hunt 2004). High prevalence about 80% or more have been documented in parts of China and some South American and Eastern European states (Roberts et al. 2016; Graham et al. 1991). H. pylori has been grouped under class I carcinogen by International Agency for Research on Cancer (Covacci et al. 1999). It is the only bacterium linked with gastric malignancy (IARC 1994), estimated to be the cause of 60% of gastric cancer cases (Parkin 2006). Other than gastric diseases, H. pylori is also associated with MALT (mucosa-associated lymphoid tissue lymphoma), vitamin B1 deficiency, iron deficiency, and idiopathic thrombocytopenic purpura (Kuipers 1997). For the prevention of H. pylori-associated complications, inhibition of infection is pivotal. Combinations of several treatments are available; triple therapy, including antibiotics and a proton pump inhibitor, is widely used (Toracchio et al. 2000). Increasing incidence of resistant H. pylori strains to antibiotics including clarithromycin and metronidazole reduces the effectiveness of triple therapy (Graham 1998). The resistance of H. pylori strains differs worldwide, varying from 10 to 90% for metronidazole and 0 to 15% for clarithromycin (Toracchio et al. 2000). Moreover, the adverse effects of antibiotics such as diarrhea, nausea, and vomiting and expensive nature of the treatment have led to the reduced compliance rate of the patients. H. pylori infection in childhood may persist through life if not treated (Mcnulty et al. 2012; Arslan et al. 2017). Despite the fact that most infected individuals remain asymptomatic, its eradication is important as it may cause chronic gastritis, dyspepsia, and gastroduodenal ulcers (Smith et al. 2014). Considering the declining efficacy of triple therapy due to increasing resistance of H. pylori to antibiotics, adverse effect of the antibiotics, patients’ non-compliance, and cost of the treatment regime, search for a better and safe alternative approach is critically needed. Probiotics have been extensively explored as an adjunct to antibiotics treatment for H. pylori infection (Patel et al. 2014). Different studies have described the therapeutic potential of probiotics to effectively cure several gastric diseases (Goderska et al. 2018; Behnsen et al. 2013; Sarowska et al. 2013).
Probiotics are defined as “living micro-organisms which provide beneficial effect on the host’s health when administered in adequate amount” (Ruggiero 2014). There are many microbial species that could potentially function as probiotics, like Lactobacillus, Bifidobacteria, Saccharomyces, Streptococcus etc., of which Lactobacillus and Bifidobacteria are the most commonly studied. Probiotics stabilize the intestinal microflora by inhibiting pathogens, which is mostly attributed to their competitiveness for food and binding sites (Denev 2006), production of antimicrobial substances, and immunomodulation (Isolauri et al. 2001). Beside antagonistic properties of probiotics, their abilities to survive high pH and bile salts and to colonize gastrointestinal surfaces are critical to assign them among the most promising and potential probiotic candidates. These properties have attracted researchers’ interest to investigate new strains and gain insight into their beneficial properties (Holzapfel et al. 2001). Numerous studies related to the antagonistic activity of probiotics against H. pylori have shown promising results in reducing antibiotic side effects, improving eradication of H. pylori infection and reducing cell injury (Lesbros-Pantoflickova et al. 2007; Wilhelm et al. 2011; Patel et al. 2014). Despite the fact that every probiotic strain is not beneficial to improve H. pylori eradication treatment, several probiotics appear to mitigate the disease and side effects of the treatment. In an assessment study of H. pylori infection after its eradication by conventional therapy in children, 30% of the children were found to be re-infected after 2 years (Magistà et al. 2005); considering this, use of probiotics as an eradication adjunct or as a vaccine delivery tool would be very useful. In the previous literatures, potential of probiotics against H. pylori in in vitro, in vivo, and clinical trials has been described without providing much knowledge about their potential as an effective vaccine delivery vehicle. In this review, we have highlighted all the potentialities of probiotics against H. pylori from their mechanism of action, preclinical and clinical journey to their use in vaccines with successful examples. Limitations in the above mentioned potentials and suggestions for the future studies have been summarized as well.
Helicobacter pylori: pathogenesis
All H. pylori-infected individuals are not likely to develop peptic ulcers. H. pylori colonize the stomach for years and cause continuous infection, but only minority show symptoms. Right after H. pylori colonization to the epithelial tissues of the stomach, the activation of the host’s innate and adaptive immune response takes place (Cadamuro et al. 2014). Prolonged existence of chronic inflammation by H. pylori may advance to atrophic gastritis, dysplasia, metaplasia, and ultimately gastric carcinoma (Fox and Wang 2007). Studies have shown that genotypic and phenotypic variance in H. pylori strains is mainly responsible for different clinical outcomes (Blaser and Berg 2002). H. pylori colonization and the successful onset of pathogenesis usually take place in steps, such as survival in low pH, movement towards epithelium mediated by flagella, strong interaction with host cell receptors, and release of several toxins (Kao et al. 2016). Primarily, the infection is developed upon H. pylori’s adhesion to the gastric mucosa. The persistent colonization which results in chronic inflammation is facilated by different virulence factors such as the production of urease enzyme and presence of flagella. Urease helps H. pylori survival in the low pH of the stomach by generating ammonia (Eaton et al. 1991; Marshall et al. 1990). Urease is composed of four sub-units, UreA, UreB, UreC, and UreD. UreB is the strongest immunogen of H. pylori (Corthesy-Theulaz et al. 1995) which has been studied widely in the development of anti-H. pylori vaccine (Gu et al. 2009; Zeng et al. 2015). H. pylori adheres and colonizes the host’s epithelial cells by using a number of adhesins including BabA, SabA, HopZ (Odenbreit et al. 2002), AlpA/B (Peck et al. 1999), urease etc. BabA and SabA bind to fucosylated and sialylated blood group antigens. Studies have shown SabA-mediated activation of neutrophils (Unemo et al. 2005). Neutrophils upon activation, either by H. pylori soluble factors or as a result of inflammation, further produce ROS which cause epithelial cell DNA damage leading to apoptosis (Bagchi et al. 1996). Urease as an adhesin attaches to MHC class II and CD74 on antigen presenting cells which induce apoptosis of the epithelial cells and stimulate secrection of IL-8 (Fan et al. 2000; Barrera et al. 2005). Thus, attachment of H. pylori to the gastric mucosal layer induces inflammation resulting in the mucosal surface injury due to the release of different cytokines and chemokines (Engstrand et al. 1989; Peek Jr et al. 1995). Production of urease enzyme and flagellated structure of H. pylori are important virulent factors for successful colonization, which is present in almost all strains. As mentioned above, not all infected individuals develop an ulcer or other complications of H. pylori, which is due to variation in virulence of different H. pylori strains. Among different virulence factors VacA and CagA, toxin genes are the main virulence factors expressed in specific H. pylori strains. CagA is a part of pathogenecity island (CagPAI). Some of the CagA genes code for T4SS (type IV secretory system) which plays an important role in injecting bacterial components into the host’s epithelium leading to the stimulation of macrophages which further triggers the production of IL-8 and INF-γ ultimately disrupt the epithlial barrier (Boonyanugomol et al. 2011). These changes further cause epithelial damage leading to tumor formation. Several studies reported H. pylori-mediated over-expression of IL-8 in the host to be significantly linked with stomach cancer (Lee et al. 2013; Macrì et al. 2006). It has been observed that individuals with CagA+ H. pylori infection more often develop severe gastric disease and cancer (Kusters et al. 2006). VacA or vacuolating toxins form vacuoles in the host’ s epithelial cells and cause disruption in membrane potential. Mitochondrial membrane potential is also disrupted leading to apoptosis (Cover et al. 2003). VacA alter antigen presentation by B cells and inhibit T cell proliferation making it an important virulence factor to establish chronic infection (Cover and Blanke 2005). VacA is present in all H. pylori strains, but its pathogenecity depends on it genotypes (Winter et al. 2014). Despite the stimulation of H. pylori-mediated immune response, the infection persists which is mainly associated with its potential to evade host’s inflammatory response. H. pylori avoids TLRs (toll-like receptors) mediated recognition by modulating its LPS and flagellin surface proteins (Cullen et al. 2011). O-antigen on the bacterial outer polysachharide resembles human blood group antigens. This molecular mimicry of H. pylori protects it from recognized by the TRLs. Modification of lipid A portion of LPS alters the net charge of its surface resulting in inability of CAMP (cationic antimicrobial peptide) to bind to its surface (Cullen et al. 2011). Besides modulation of LPS, H. pylori’s LPS loosely binds to its host’s receptor which results in reduced activation of immune cells (Sutton and Chionh 2013). Different studies have also suggested that CagA and VacA virulence genes protect H. pylori from phagocytic cells (Ramarao et al. 2000; Zheng and Jones 2003). H. pylori also stimulates expansion of regulatory T cell (Treg) which downregulate inflammatory response by actively modulating the differentiation of dendritic cells and T cells (Beswick et al. 2007; Lundgren et al. 2003) (Fig. 1).
How probiotics work against H. pylori
Several experimental studies have been able to propose various possible probiotic’s antagonistic effect on H. pylori, though the precise mechanisms have yet to be uncovered. Probiotic’s abilities to compete for binding receptors, modulate immunity, strengthen the mucosal barrier, and co-aggregate the pathogens are generally attributable to their effectiveness against various pathogens.
The mucosal barrier
The epithelium lining the gastrointestinal mucosa acts as a powerful barrier for the pathogens. Intestinal epithelial cells being the primary cell type to encounter the invading pathogens provide the first line of defense against harmful organisms. Upon invasion of pathogens, epithelial cells initiate an innate immune response which stimulates the secretion of chemokines and cytokines that connect the innate and adaptive immune response. Additionally, epithelial cells also produce mucus layer, which further provides protection to the mucosal surfaces from pathogens. Disruption of the mucosal barrier leads to different disease conditions. Probiotics can positively affect the epithelial barrier function which is strain specific (Seth et al. 2008; Karczewski et al. 2010). H. pylori damages the gastric mucosa using its virulence factors, like CagA and VacA (Backert et al. 2016). In cases of gastritis caused by H. pylori, decreased mucus secretion in a damaged epithelium has been observed (Lesbros-Pantoflickova et al. 2007). Moreover, in a study with human gastric cell line, H. pylori suppressed MUCI and MUC5A gene expression (Byrd et al. 2000) and caused disruption of the mucosal barrier, as mucins being high molecular weight glycoproteins are useful for gastric epithelium stability.
Probiotics protect the mucosal barrier from damage by different mechanisms including modification of the expression of mucus and epithelial junction proteins and releasing bioactive molecules to stabilize the barrier, thus preventing its disruption by the pathogens. Different studies demonstrated the increased production of IgA by probiotic strains, which is helpful in strengthening the mucosal barrier against pathogen invasion (Perdigón et al. 2000; Viljanen et al. 2005). As seen in in vitro studies, L.plantarum strain 299v and L.rhamnosus GG enhance the MUC2 and MUC3 gene expression providing strength to the mucus barrier (Mack et al. 1999). Another study on H. pylori gastritis proves increased thickness of mucus layer upon intake of L. johnsonii in fermented milk (Pantoflickova et al. 2003). Bergonzelli et al. (2006) reported an efficient binding of recombinant GroEL from Lactobacillus johnsonii LA1 to the HT29 cells and hypothsized its potential in pathogens’ exclusion.
Competition for adhesion
H. pylori binds to the gastric epithelium in order to colonize and initiate infection. Probiotics ability to prevent H. pylori from binding to the epithelial cells usually brought about by different mechanisms such as competing for the adhesion sites or nutrients, causing steric hindrance and secreting antimicrobial substances. Several reports describe the adhesion of probiotics to the specific binding receptors, L. reuteri was found to compete for specific binding receptor site asialo-GMI and sulfatide and inhibit H. pylori adhesion (Mukai et al. 2002). Another study reports affinity of S. boulardii to sialic acid receptor followed by inhibition of H. pylori from binding (Sakarya and Gunay 2014). Some Lactobacilli strains such as L. acidophilus LB (Coconnier et al. 1998) and L. johnsonii La1 (Michetti et al. 1999) secrete antimicrobial substances to inhibit attachment of H. pylori to the epithelium. Competitive exclusion of H. pylori by potential probiotic strains is also evident by several in vitro studies with L. acidophilus LB (Coconnier et al. 1998), L. johnsonii (Michetti et al. 1999), L. salivarius (Kabir et al. 1997), and W. confusa (Nam et al. 2002). W. confusa strain PL9001 significantly inhibits H. pylori from binding to the gastric cell lines (Nam et al. 2002). Some studies provide evidence of reduced H. pylori colonization in germ-free mice which were previously colonized by probiotics (Kabir et al. 1997; Johnson-Henry et al. 2004). Anti-adhesion property of probiotics is one of the crucial mechanisms to counteract pathogens from invading the host. It would be interesting to investigate the underlying molecular mechanism of probiotics for their increased affinity towards the binding receptors.
Secretion of antimicrobials
H. pylori survival in the acidic environment of the stomach is mediated by urease production, which increases the pH of the gastric surrounding by converting urea into ammonia and CO2. Probiotics secrete antimicrobials as a result of fermentation, such as lactic acid, acetic acid, and hydrogen peroxide (Vandenbergh 1993). Lactic acid secreted by probiotic lowers down the surrounding pH making it unfavorable for H. pylori’s growth (Aiba et al. 1998; Midolo et al. 1995; Sgouras et al. 2004). Besides lowering the pH, lactic acid was also found to have inhibitory activity against urease (Sgouras et al. 2004). A number of authors have reported the inhibitory action of lactic acid produced by Lactobacilli against H. pylori, for example, L. casei subsp. Rhamnosus and L. acidophilus (Midolo et al. 1995; Bhatia et al. 1989).
It is worth noting that not all lactic acid-producing Lactobacilli are capable of anti-Helicobacter pylori activity, as L. johnsonii La10, despite producing lactic acid, does not show inhibition of H. pylori, whereas L. johnsonii La1 does (Michetti et al. 1999). This also suggests that antagonistic activity of Lactobacilli against H. pylori is strain specific. Other antimicrobial products have also been reported to have antagonistic effect against H. pylori. Culture supernatant of L. johnsonii La1 (Michetti et al. 1999) and L. acidophilus LB (Coconnier et al. 1998) effectively inhibits H. pylori in in vitro as well as in mice. Lorca et al. (2001) described inhibition of H. pylori, mediated by autolysin of L. acidophilus CRL 639, and suggested that it released after cell lysis. Strong inhibitory activity against H. pylori has been observed by lacticins A164 and BH5 of L. lactis subsp. A164 and BH5 (Kim et al. 2003). The exact nature of these antimicrobial substances has not been investigated. Bacteriocins are proteinaceous antimicrobial peptides which have been studied extensively (Cotter et al. 2013). Bacteriocin production by probiotics has been considered as one of their most essential properties (Dobson et al. 2011). Bacteriocin-mediated inhibition of H. pylori has been reported by Bacillus subtilis (Pinchuk et al. 2001) and W. confusa (Nam et al. 2002). Inhibition by B. subtilis was shown by animocumacins, grouped under isocoumarin antibiotics (Pinchuk et al. 2001). de Klerk et al. (2016) demonstrated direct action of L. gasseri Kx110A1 and L. brevis ATCC14869 conditioned medium on H. pylori and reported a reduction in SabA gene expression mediated by an unknown effector molecule. The authors suggested the effector molecule to be either an anti-microbial substance or a bacterial surface molecule released into the conditioned medium. Reuterin from L. reuteri ATCC 55730 reported to inhibit VacA gene expression of H. pylori (Urrutia-Baca et al. 2017).
SabA and VacA are important virulence factors of H. pylori, inhibition of their expression is critically important to regulate inflammation and prevent tumor formation.
Immunomodulation mechanism
H. pylori infection stimulates inflammation, and several inflammatory mediators like cytokines, chemokines etc. are released. Interleukin 8 (IL-8) triggers the secretion of neutrophils and monocytes to the gastric mucosal surfaces. Following this, the dendritic cells and monocytes activate the secretion of TNF-α, IL-1, and IL-6 (Noach et al. 1994). The stimulation of CD 4 + T cells (type 1) by IL-1 and IL-6 produces various cytokines such as IL-4, -5, -6, and IFN-γ (Harris et al. 1996); however, the H. pylori infection prevails. Immunomodulation is a well-known characteristic of probiotics. They interact with gastric epithelial cells and reduce the inflammation and gastric activity as a result of secretion of anti-inflammatory cytokines (Wiese et al. 2012). Experimental studies in mice reported a reduction in IgG immunoglobulins specific to H. pylori infection after probiotic intake (Aiba et al. 1998; Sgouras et al. 2004). The culture supernatant of L. acidophilus strain LB effectively reduces H. felis density, urease activity, and cures the inflammation in mice (Coconnier et al. 1998). L. casei strain Shirota decreased H. pylori-mediated inflammatory response in experimental mice (Sgouras et al. 2004). The strength of probiotics to weaken the H. pylori infection and inflammatory response varies from strain to strain. This can be exemplified in a study in which L. salivarius significantly reduced inflammation caused by H. pylori in gnotobiotic mice as compared to L. acidophilus or L. casei (Aiba et al. 1998). Lactic acid produced by probiotics has been shown to reduce inflammation by regulating inflammatory cytokines in several animal studies (Coconnier et al. 1998; Murosaki et al. 2000). CagA virulence gene of H. pylori has been strongly linked with increased gastric malignancy (Blaser and Berg 2002) which is suggested to be due to CagA-mediated enhanced IL-8 levels in the gastric mucosa (Peek Jr et al. 1995). Some probiotics L. bulgaricus (Zhou et al. 2008), L. acidophilus (Yang et al. 2012), and L. salivarius (Kabir et al. 1997) have been reported to down-regulate IL-8 secretion by H. pylori. This characteristic of some probiotic strains would open doors to discover new strategies to manage gastric cancers. Variations in immunomodulation process have been observed in different probiotic strains which may be due to polymorphism in the host’s immunity (Noach et al. 1994), which is a complex process to extrapolate. Thus, it is evident from various animal studies that probiotics are significantly effective to reduce the degree of inflammation and outcome of H. pylori infection.
Co-aggregation and aggregation
Co-aggregation is the binding of organisms of diverse species, while aggregation or auto aggregation is the attachment of the organisms of similar species (Rickard et al. 2003; Schembri et al. 2001). Exclusion of pathogens binding to the intestinal mucosa as a result of aggregating property of probiotics has been described previously (Tareb et al. 2013). L. reuteri DSM17648 significantly co-aggregated with H. pylori in in vitro and in vivo studies (Holz et al. 2015). Similarly, H. pylori inhibition by L. gasseri occurred on account of co-aggregation in vitro (Chen et al. 2010). Studies also reported aggregation of H. pylori by L. johnsonni La1 (NCC533) recombinant GroEL protein receptor in a specific manner (Bergonzelli et al. 2006). Other probiotic strains are suggested to be investigated for this property.
It is crucial to identify the precise molecular mechanism underlying probiotics action on health and disease conditions. Once identified in vitro efficacy, their potential in physiological conditions is equally important (Fig. 2, Table 1).
Clinical studies
Numerous clinical studies have been documented to investigate the anti-H. pylori activity of probiotics and their potential to ameliorate the antibiotic-associated side effects. (Table 2, Table 3). Different clinical researches with probiotic strain L. johnsonii La1 have been described (Felley et al. 2001; Pantoflickova et al. 2003; Ojetti et al. 2012; Michetti et al. 1999). The strain was administered either as a live bacterium added in fermented milk or as a cell-free culture supernatant (Michetti et al. 1999). All results showed a significant reduction in H. pylori density. L. acidophilus when administered as a cell-free culture supernatant showed anti-urease activity in asymptomatic individuals (Coconnier et al. 1998). In clinical studies, probiotics are generally examined either as an alternative or an adjunct to antibiotics.
Potential of probiotics as an alternative to antibiotics
A double-blind controlled clinical study including 252 asymptomatic children previously tested by C-urea breath test as H. pylori positive has been conducted (Cruchet et al. 2003). The children were arranged in groups and administered with live L. johnsonii La1, heat-killed L. johnsonii La1, live L. paracsei ST11, heat-killed L. paracasei ST11 daily for a month. At the end of the trial period, only children who received live L. johnsonii La1 showed a significant reduction in urease activity as compared to the other groups. Similarly, Wang et al. (2004) demonstrated a decrease in H. pylori colonization and gastritis in dyspeptic patients after ingestion of L. acidophilus La5 and B. lactis Bb12 containing yoghurt. Gotteland et al. (2005) investigated L. acidophilus or S.boulardii plus inulin effect on H. pylori-infected children and also compared the effect as an adjunct to standard triple therapy. Significant decrease in urease activity in inulin group was observed; however, H. pylori eradication rate with L. acidophilus and inulin group was not significant (6.5% and 12%, respectively) as compared to standard triple therapy (66%). Similarly, Francavilla et al. (2014) administered a daily dose of L. reuteri mixture and placebo to the groups of 50 patients each. L. reuteri group showed 75% eradication rate whereas 65% of placebo were eradicated. Few patients receiving L. reuteri mixture reported side effects as compared to placebo. Different probiotic strains L. johnsonii La1 (Gotteland and Cruchet 2003), L. gasseri OLL 2716 (Sakamoto et al. 2001), L. reuteri ATCC 55730 (Francavilla et al. 2008), and B. bifidus BF-1(Miki et al. 2007) as single therapy did not eradicate H. pylori in adults rather modulate its colonization. Different level of efficacy is due to different strains of probiotics tested. Further studies are required to address the efficacy of anti-H. pylori property of probiotics and evaluation of the specific immune mechanism involved in probiotics immunomodulation is suggested to provide scientific evidence for the clinical benefit of individual probiotic strain (Table 2).
Potential of probiotics as an adjunct to antibiotics
Studies on the effect of probiotics in alleviating the side effects of standard H. pylori treatment have been increasing, usually owing to their usefulness in increasing the patient’s compliance rate (Goderska et al. 2018). Ojetti et al. (2012) investigated the effect of co-administration of L. reuteri ATCC 55730 with antibiotics on H. pylori-infected subjects, which significantly increased H. pylori eradication rate; additionally, the adverse effects of antibiotics were also reduced. In the same way, Myllyluoma et al. (2005) reported a significant reduction in H. pylori load and gastritis after treating the patients with a combination of probiotics as a complement to H. pylori treatment. A study by Du et al. (2012) demonstrated improvement in H. pylori eradication when L. acidophilus was used as a supplement to triple therapy; however, symptoms were not reduced with probiotic alone. No significant eradication was observed upon administration of L. reuteri ATCC 55730, though side effects were reduced to some extent (Lionetti et al. 2006). Similar results were observed in a study by Armuzzi et al. (2001) in which L. rhamnosus GG was supplemented as a complementary therapy. In view of the potential of probiotics against H. pylori infection, researchers have also examined the combination of different species of probiotics complementary to the triple therapy. In a double-blind placebo-controlled study, 66 H. pylori-infected children were administered combination of probiotics including L. rhamnosus, L. acidophilus, L. bulgaricus, L. casei, S. thermophilus, B. breve, and B. infantis along with triple therapy. A total of 90.09% of the children supplemented with probiotics as an adjunct to antibiotic therapy were successfully cured from H. pylori infection whereas 69.69% of children in the control group receiving placebo were cured. The significant rise of approximately 20% in eradication rate of the treated group remarkably proves the efficacy of probiotics as an adjunct to H. pylori eradication therapy (Ahmad et al. 2013). Emara et al. (2014) demonstrated the administration of a mixture of L. reuteri DSM 17938 and L. reuteri ATCCPTA 6475 as a complementary therapy. After the treatment, patients were re-examined for the presence of H. pylori antigen in stool, and histology of the biopsy specimen was carried out. Increased eradication from H. pylori infection was detected with reduced adverse effects of the eradication therapy and improved histology of H. pylori as compared to placebo group. Contrastingly, no effect had been observed upon co-ingestion of probiotic containing yoghurt with antibiotic treatment for H. pylori infection in children (Goldman et al. 2006) (Table 3).
Meta-analyses on the available outcomes of clinical trials using probiotics as a therapeutic agent are useful to understand the vitality and drawbacks of the clinical research. Recently, Feng et al. (2017) compared the potential of 17 probiotics as an adjunct to triple therapy versus as a sole therapy and found L. casei to be the most potent probiotic used as monotherapy, whereas L. casei, L. plantarum, L. acidophilus, L. reuteri, L. rhamnosus, L. salivarius, L. sporogenes, B. infantis, B. longum, and S. thermophilus as a multi-species probiotic combination showed promising result in reducing treatment-related side effects. Zheng et al. (2013) conducted a meta-analysis of 9 RCT (randomized controlled trial) including 1163 patients. They compared the potential of probiotic supplements as an adjunct to triple therapy or sequencial therapy with that of placebo. Upon comparing the outcome of probiotics intervention, they found 78.18% eradication in the treated group and 68.54% eradication in the placebo (control) group, showing approximately 10% increase in the eradication rate of the treated group. However, decrease in the side effect was not significant (31.21% decrease in the treated group vs 34.86% decrease in the control group). In the same study, subgroup analysis of five trials showed significant increase of 17% in eradication rate of the treated group when compared to the control group upon administering Lactobacillus species only, whereas only 2.8% eradication was observed when multi-strain probiotics were supplemented. It was concluded that Lactobacillus containing probiotic may have enhanced benefits as compared to combination of species of probiotics. In a comprehensive study, Wang et al. (2017) compared the potential of probiotic supplement for H. pylori eradication, most probiotics were successful in eradicating the H. pylori infection, while single probiotic strain showed improved result as compared to multi strain therapy. Contrastingly, Dang et al. (2014) found a probiotic supplement to be active in H. pylori eradication only when the antibiotic treatment failed.
In a recent study co-administeration of L. casei rhamnosus (LCR 35) effectively reduced antibiotic side effects but no significant difference in the eradication rate in the tested and control group was observed. The authors suggest that the low difference may be due to the use of better antibiotic regime (Uitz et al. 2017). McNicholl et al. (2018) conducted a controlled double-blind clinical trial with two probiotic strains L. plantarum and Pediococcus acidilactici, which were previously found effective in vitro in other studies (Kaur et al. 2014; Sunanliganon et al. 2012). No success in the improvement of the side effect and H. pylori colonization was observed. This clearly suggests that clinical trials are crucial to evaluate potential of the probiotic strain tested in vitro. B. animalis subsp. lactis significantly increase the eradication rate with decreased side effects upon its co-administration with conventional antibiotics (Çekin et al. 2017).
The differing results, though apparently indicate probiotics potential against H. pylori eradication, may be due to many factors related to deviating experimental design and setup. Thus, consistency in experimental protocol with a defined combination of probiotic supplement would be useful to get accuracy in the outcome.
Vaccine development
Efforts in the development of vaccines against H. pylori started soon after its discovery by Marshall. BJ and Warren RM (Marshall and Warren 1984). The gastric cancer due to this notorious bacterium is the main reason of establishing a potent vaccine, as it is the third major cancer causing agent worldwide (IARC 2012). The two main approaches are prophylactic and therapeutic administration of the vaccines. As the infection is usually contracted at an early age (Mitchell et al. 1992), prophylactic immunization of children is crucial. Recently, a phase 3 clinical trial in China has been reported in which recombinant urease B vaccine successfully immunized 70% of the children (Zeng et al. 2015). This is a breakthrough in the development of potent vaccine prompting further research in this field.
The second approach is a therapeutic vaccine which can be given at any period; however, stimulation of immunosupressive mechanism by H. pylori to establish chronic infection is a major challenge. Therapeutic protections in mice have been reported previously in different studies (Doidge et al. 1994; Sutton et al. 2000); hence, its efficacy in human is yet to be achieved.
Probiotics as vaccine delivery system for H. pylori infection
With the advancement in the field of genetic engineering, probiotics have emerged as a useful tool to deliver vaccines. Many probiotic organisms are considered GRAS (generally regarded as safe) by the Food and Drug Administration. Owing to their GRAS nature, they are widely used in food industry. Lactococci have been suggested as an ideal recombinant vaccine vehicle, mostly due to their potential to induce both acquired and innate immunity in the host. Production of recombinant H. pylori antigens like UreB, CagA, NapA etc. have been widely demonstrated in L. lactis followed by their efficacy in pre-clinical studies which showed varied outcomes (Gu et al. 2009; Lee et al. 2001; Kim et al. 2009). Previously, in an attempt to develop H. pylori vaccine, we have successfully expressed Ure-B antigen in L. lactis. The recombinant L. lactis produced significant anti-Ure B serum antibody and protected the mice against gastritis (Gu et al. 2009). We detected increase in IgG level, while IgA specific to Ure-B were detected in fresh feces which declined after 38 days. In a similar experiment by Lee et al. (2001), no immunization has been observed, upon administration of recombinant Ure-B L. lactis to H. pyloi SS1 challenged mice. Interestingly, Ure-B-specific serum IgG were detected. This suggests that presence of both IgG and IgA is important to elicit effective immune response. In order to study surface display expression of H. pylori antigens in probiotics, we successfully constructed a recombinat UreBE-SpaxX (Ure B fragment E and fragment Spax of Staphylococcus aureus) (Song and Gu 2009). SpaxX is a cell wall anchor of S. aureus and its fusion with Ure BE would provide enhanced adjuvant activity. Western blotting of the recombinant L. lactis cell wall extract with polyclonal chicken antiserum confirmed its effecacy. Bacillus subtilis spores were used to deliver recombinant urease B antigen, which significantly reduced H. pylori load (84%) in mice (Zhou et al. Ahmad K, Fatemeh F, Mehri N, Maryam S (2013) Probiotics for the treatment of pediatric Helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr 23:79–84 Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y (1998) Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivariusas a probiotic in a gnotobiotic murine model. Am J Gastroenterol 93(11):2097–2101 Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, Santarelli L, Cammarota G, De Lorenzo A, Pola P, Gasbarrini G, Gasbarrini A (2001) Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 63:1–7 Arslan N, Yılmaz O, Demiray-Gürbüz E (2017) Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol 23(16):2854–2869 Backert S, Neddermann M, Maubach G, Naumann M (2016) Pathogenesis of Helicobacter pylori infection. Helicobacter 21(Suppl 1):19–25 Bagchi D, Bhattacharya G, Stohs SJ (1996) Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res 24:439–450 Barrera CA, Beswick EJ, Sierra JC, Bland D, Espejo R, Mifflin R, Adegboyega P, Crowe SE, Ernst PB, Reyes VE (2005) Polarized expression of CD74 by gastric epithelial cells. J Histochem Cytochem 53:1481–1489 Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M (2013) Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med 3:A010074 Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthésy-Theulaz IE (2006) GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun 74:425–434 Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE (2007) Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun 75:4334–4341 Bhatia SJ, Kochar N, Abraham P, Nair NG, Mehta AP (1989) Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol 27:2328–2330 Blaser MJ, Berg DE (2002) H.pylori and genetic diversity and risk of human disease. J Clin Invest 2:28–37 Boonyanugomol W, Chomvarin C, Baik SC, Song JY, Hahnvajanawong C, Kim KM, Cho MJ, Lee WK, Kang HL, Rhee KH, Sripa B (2011) Role of cagA-positive Helicobacter pylori on cell proliferation, apoptosis, and inflammation in biliary cells. Dig Dis Sci 56:1682e92 Byrd JC, Yunker CK, Xu QS, Sternberg LR, Bresalier RS (2000) Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterol 118:1072–1079 Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE (2014) Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol 20:1424–1437 Çekin AH, Şahintürk Y, Akbay Harmandar F, Uyar S, Yolcular BO, Çekin Y (2017) Use of probiotics as an adjuvant to sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turk J Gastroenterol 28:3–11 Chen X, Tian F, Liu X, Zhao J, Zhang HP, Zhang H, Chen W (2010) In vitro screening of lactobacilli with antagonistic activity against Helicobacter pylori from traditionally fermented foods. J Dairy Sci 93(12):5627–5634 Coconnier MH, Lievin V, Hemery E, Servin AL (1998) Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol 64:4573–4580 Corthesy-Theulaz I, Porta N, Glauser M, Sarage E, Vaney AC, Haas R, Kraehenbuhl JP, Blum A, Michetti P (1995) Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterol 109:115–121 Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative toantibiotics? Nat Rev Microbiol 11(2):95–105 Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R (1999) Helicobacter pylori virulence and genetic geography. Science 284:1328–1333 Cover TL, Blanke SR (2005) Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol 3:320–332 Cover TL, Krishna US, Israel DA, Peek RM Jr (2003) Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res 63:951–995 Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M (2003) Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 19:716–721 Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS (2011) Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7:e10024 Dang Y, Reinhardt JD, Zhou X, Zhang G (2014) The effect of probiotic supplementation on Helicobacter pylori eradication therapy: a meta-analysis. PLoS One 9(11):1–15 de Klerk N, Maudsdotter L, Gebreegziabher H, Saroj SD, Eriksson B, Eriksson OS, Roos S, Lindén S, Sjölinder H, Jonsson AB (2016) Lactobacilli reduce Helicobacter pylori attachment to host gastric epithelial cells by inhibiting adhesion gene expression. Infect Immun 84:1526–1535 Denev SA (2006) Role of Lactobacilli in gastrointestinal ecosystem. Bulg J Agric Sci 12(1):63–114 Dobson A, Cotter PD, Ross RP, Hill C (2011) Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78(1):1–6 Doidge C, Crust I, Lee A, Buck F, Hazell S, Manne U (1994) Therapeutic immunisation against Helicobacter infection. Lancet 343:914–915 Du YQ, Su T, Fan JG, Lu YX, Zheng P, Li XH, Guo CY, Xu P, Gong YF, Li ZS (2012) Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pyloriinfection. World J Gastroenterol 18:6302–6307 Dunne C, Dolan B, Clyne M (2014) Factors that mediate colonization of the human stomach by Helicobacter pylori. World J Gastroenterol 20:5610–5624 Eaton KA, Brooks CL, Morgan DR, Krakowka S (1991) Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun 59:2470–2475 Emara MH, Mohamed SY, Abdel-Aziz HR (2014) Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Ther Adv Gastroenterol 7:4–13 Engstrand L, Scheynius A, Påhlson C, Grimelius L, Schwan A, Gustavsson S (1989) Association of Campylobacter pylori with induced expression of class II transplantation antigens on gastric epithelial cells. Infect Immun 57:827–832 Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE (2000) Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol 165:1918–1924 Felley CP, Corthesy-Theulaz I, Rivero JL, Sipponen P, Kaufmann M, Bauerfeind P, Wiesel PH, Brassart D, Pfeifer A, Blum AL, Michetti P (2001) Favourable effect of acidified milk (LC-1) on Helicobacter pylori gastritis in man. Eur J Gastroenterol Hepatol 13:25–29 Feng JR, Wang F, Qiu X, McFarland LV, Chen PF, Zhou R, Liu J, Zhao Q, Li J (2017) Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol 65:231–238 Fox JG, Wang TC (2007) Inflammation, atrophy, and gastric cancer. J Clin Invest 117:60–69 Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E, Miniello VL (2008) Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter 13:127–134 Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A, Cavallo L, Francavilla A, Versalovic J (2014) Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol 48:407–413 Goderska K, Agudo Pena S, Alarcon T (2018) Helicobacter pylori treatment: antibiotics or probiotics. J Appl Microbiol Biotechnol 102(1):1–7 Goldman CG, Barrado DA, Balcarce N, Rua EC, Oshiro M, Calcagno ML, Janjetic M, Fuda J, Weill R, Salgueiro MJ, Valencia ME, Zubillaga MB, Boccio JR (2006) Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition 10:984–988 Gotteland M, Cruchet S (2003) Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother 51:1317–1319 Gotteland M, Poliak L, Cruchet S, Brunser O (2005) Effect of regular ingestion of Saccharomyces boulardii plus inulin orLactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr 94:1747–1751 Graham DY (1998) Antibiotic resistance in Helicobacter pylori: implication for therapy. Gastroenterology 115:1272–1277 Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E (1991) Epidemiology of Helicobacter pylori in an asymptomatic population in United States: effect of age, race, and socioeconomic status. Gastroenterology 100(6):1495–1501 Gu Q, Song D, Zhu M (2009) Oral vaccination of mice against Helicobacter pylori with recombinant Lactococcus lactis expressing urease subunit B. FEMS Immunol Med Microbiol 56(3):197–203 Harris PR, Mobley HL, Perez-Perez GI, Blaser MJ, Smith PD (1996) Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 111:419–425 Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, Dixon MF (1990) Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut 31:134–138. https://doi.org/10.1136/gut.31.2.134
Holz C, Busjahn A, Mehling H, Arya S, Boettner M, Habibi H, Lang C (2015) Significant reduction in Helicobacter pylori load in humans with non-viable Lactobacillus reuteri DSM17648: a pilot study. Probiotics Antimicrob Proteins 7:91–100 Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U (2001) Taxonomy and important features of probiotic microorganisms in food nutrition. Am J Clin Nutr 73:365S–373S IARC (1994) Schistosomes, liver flukes and Helicobacter pylori. Vol 61. International Agency for Research on Cancer. Lyon, France: IARC Press (1994) IARC Monographs on the evaluation of carcinogenic risks to humans pp.177–240 International Agency for Research on Cancer GLOBOCAN (2012) Estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organisation Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S (2001) Probiotics: effects of immunity. Am J Clin Nutr 73(2 Suppl):S444–S450 Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM (2004) Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci 49:1095–1102 Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y (1997) Prevention of Helicobacter pylori infection by Lactobacilli in a gnotobiotic murine model. Gut 41:49–55 Kao CY, Sheu BS, Wu JJ (2016) Helicobacter pylori infection: an overview of bacterial virulence factors and pathogenesis. Biom J 39(1):14–23 Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarumin vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298(6):G851–G859 Kaur B, Garg N, Sachdev A, Kumar B (2014) Effect of the oral intake of probiotic Pediococcus acidilactici BA28 on Helicobacter pylori causing peptic ulcer in C57BL/6 mice models. Appl Biochem Biotechnol 172:973–983 Kim TS, Hur JW, Yu MA, Cheigh CI, Kim KN, Hwang JK, Pyun YR (2003) Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot 66:3–12 Kim SJ, Lee JY, Jun DY, Song JY, Lee WK, Cho MJ, Kim YH (2009) Oral administration of Lactococcus lactis expressing Helicobacter pylori Cag7-ct383 protein induces systemic anti-Cag7 immune response in mice. FEMS Immunol Med Microbiol 57:257–268 Kuipers EJ (1997) Helicobacter pyloriand the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther 11(1):71–88 Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19(3):449–490 Lee MH, Roussel Y, Wilks M, Tabaqchali S (2001) Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H.pylori infection in mice. Vaccine 19:3927–3935 Lee KE, Khoi PN, **a Y, Park JS, Joo YE, Kim KK, Choi SY, Jung YD (2013) Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol 19(45):8192–8202 Lesbros-Pantoflickova D, Corthe’Sy-Theulaz I, Blum AL (2007) Helicobacter pylori and probiotics. J Nutr 137:812S–818S Lionetti E, Miniello VL, Castellaneta SP, Magista AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R (2006) Lactobacillus reuteritherapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther 24:1461–1468 Lorca GL, Wadström T, Valdez GF, Ljungh A (2001) Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr Microbiol 42:39–44 Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS (2003) Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun 71:1755–1762 Lv X, Song H, Yang J, Li T, ** T, **ng Y (2014) A multi-epitope vaccine CTB-UE relieves Helicobacter pylori-induced gastric inflammatory reaction via up-regulating microRNA-155 to inhibit Th17 response in C57/BL6 mice model. Hum Vaccin Immunother 10:3561–3569 Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Phys 276:G941–GG50 Macrì A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C (2006) Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers 11:184–193 Magistà AM, Ierardi E, Castellaneta S, Miniello VL, Lionetti E, Francavilla A, Ros P, Rigillo N, Di Leo A, Francavilla R (2005) Helicobacter pylori status and symptom assessment two years after eradication in pediatric patients from a high prevalence area. J Pediatrics Gastroenterol Nutr 40:312–318 Marshall BJ, Warren RM (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 16:1311–1315 Marshall BJ, Barrett LJ, Prakash C, McCallum RW, Guerrant RL (1990) Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 99:697–702 McNicholl AG, Molina-Infante J, Lucendo AJ, Calleja JL, Pérez-Aisa Á, Modolell I, Aldeguer X, Calafat M, Comino L, Ramas M, Callejo Á, Badiola C, Serra J, Gisbert JP (2018) Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: a randomized, double-blind, placebo-controlled trial. Helicobacter 23:e12529 Mcnulty CA, Lasseter G, Shaw I, Nichols T, D’Arcy S, Lawson AJ, Glocker E (2012) Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment Pharmacol Ther 35:1221–1230 Michetti P, Dorta G, Wiesel PH, Brassart D, Verdu E, Herranz M, Felley C, Porta N, Rouvet M, Blum AL, Corthésy-Theulaz I (1999) Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion 60:203–209 Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML (1995) In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol 79(4):475–479 Miki K, Urita Y, Ishikawa F, Iino T, Shibahara-Sone H, Akahoshi R, Mizusawa S, Nose A, Nozaki D, Hirano K, Nonaka C, Yokokura T (2007) Effect of Bifidobacterium bifidum fermented milk on Helicobacter pylori and serum pepsinogen levels in humans. J Dairy Sci 90:2630–2640 Mitchell HM, Li YY, Hu PJ, Liu Q, Chen M, Du GG, Wang ZJ, Lee A, Hazell SL (1992) Epidemiology of Helicobacter pylori in southern China: Identification of early childhood as the critical period for acquisition. J Infect Dis 166:149–153 Moayyedi P, Hunt RH (2004) Helicobacter pylori public health implications. Helicobacter 9(1):67–72 Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H (2002) Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol 32:105–110 Murosaki S, Muroyama K, Yamamoto Y, Yoshikai Y (2000) Antitumor effect of heat-killed Lactobacillus plantarum L-137 through restoration of impaired interleukin-12 production in tumor-bearing mice. Cancer Immunol Immunother 49:157–164 Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H, Rautelin H, Korpela R (2005) Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy a placebo-controlled, double-blind, randomized pilot study. Aliment Pharmacol Ther 21:1263–1272 Nam H, Ha M, Bae O, Lee Y (2002) Effect of Weissella confusa strain PL9001 on the adherence and growth of Helicobacter pylori. Appl Environ Microbiol 68:4642–4645 Noach LA, Bosma NB, Jansen J (1994) Mucosal tumor necrosis factor alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol 29:425–429 Odenbreit S, Faller G, Haas R (2002) Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int J Med Microbiol 292:247–256 Ojetti V, Bruno G, Ainora ME, Gigante G, Rizzo G, Roccarina D, Gasbarrini A (2012) Impact of Lactobacillus reuteri supplementation on anti-Helicobacter pylori Levofloxacin-Based Second-Line Therapy. Gastroenterol Res Pract:740381 Pantoflickova D, Corthesy-Theulaz I, Dorta G, Isler P, Rochat F, Enslen M, Blum AL (2003) Favorable effect of long-term intake of fermented milk containing Lactobacillus johnsonii on H.pylori associated gastritis. Aliment Pharmacol Ther 18:805–813 Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044 Patel A, Shah N, Prajapati JB (2014) Clinical application of probiotics in the treatment of Helicobacter pylori infection—a brief review. J Microbiol Immunol Infect 47:429–437 Peck B, Ortkamp M, Diehl KD, Hundt E, Knapp B (1999) Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res 27:3325–3333 Peek RM Jr, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ (1995) Heightened inflammatory response and cytokine expression in vivo to cagA/ Helicobacter pylori strains. Lab Investig 71:760–770 Peng X, Zhang R, Duan G, Sun N, Zhang L, Chen S, Fan Q, ** Y (2018) Production and delivery of Helicobacter pylori NapA in Lactococcus lactis and its protective efficacy and immune modulatory activity. Sci Rep 8:6435 Perdigón G, Medina M, Vintiñi E, Valdéz JC (2000) Intestinal pathway of internalization of lactic acid bacteria and gut mucosal immunostimulation. Int J Immunopathol Pharmacol 13:141–150 Phadnis SH, ParlowM H, Levy M, Ilver D, CaulkinsC M, ConnorsJ B, DunnB E (1996) Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun 64(3):905–912 Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Megraud F, Urdaci MC (2001) In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob Agents Chemother 45:3156–3161 Ramarao N, Gray-Owen SD, Backert S, Meyer TF (2000) Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol Microbiol 37:1389–1404 Rickard A, Gilbert P, High N, Kolenbrander P, Handley P (2003) Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 11(2):94–100.104 Roberts SE, Morrison-Rees S, Samuel DG, Thorne K, Akbari A, Williams JG (2016) Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol Ther 43:334–345 Ruggiero P (2014) Use of probiotics in the fight against Helicobacter pylori. World J Gastrointest Pathophysiol 5(4):384–391 Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y (2001) Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother 47:709–710 Sakarya S, Gunay N (2014) Saccharomyces boulardii expresses neuraminidase activity selective for α-2, 3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS 122:941–950 Sarowska J, Choroszy-Król I, Regulska-llow B, Frej-Mądrzak M, Jama-Kmiecik A (2013) The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv Clin Exp Med 22:759–766 Schembri M, Christiansen G, Klemm P (2001) FimH-mediated autoaggregation of Escherichia coli. Mol Microbiol 41(6):1419–1430 Seth A, Yan F, Polk DB, Rao RK (2008) Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 294(4):G1060–G1069 Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A (2004) In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol 70:518–526 Smith SM, O’Morain C, Mcnamara D (2014) Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol 20:9912–9921 Song D, Gu Q (2009) Surface expression of Helicobacter pylori urease subunit B gene E fragment on Lactococcus lactis by means of the cell wall anchor of Staphylococcus aureus protein a. Biotechnol Lett 31:985–989 Sunanliganon C, Thong-Ngam D, Tumwasorn S, Klaikeaw N (2012) Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J Gastroenterol 18:2472–2480 Sutton P, Chionh YT (2013) Why can’t we make an effective vaccine against Helicobacter pylori? Expert Rev Vaccines 12:433–441 Sutton P, Doidge C (2003) Helicobacter pylori vaccines spiral into the new millennium. Dig Liver Dis 35(10):675–687 Sutton P, Wilson J, Kosaka T, Wolowczuk I, Lee A (2000) Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol Cell Biol 78:28–30 Tareb R, Bernardeau M, Gueguen M, Vernoux J (2013) In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J Med Microbiol 62:637–649 Toracchio S, Cellini L, Di Campli E, Cappello G, Malatesta MG, Ferri A, Ciccaglione AF, Grossi L, Marzio L (2000) Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther 14:1639–1643 Uitz E, Tonninger-Bahadori K, Nekrep K, Bahadori B (2017) The effect of Lactobacillus casei rhamnosus (LCR35) supplementation on the adherence tolerance and efficiency of Helicobacter pylori: an open-label, observational, non-intervational, multicentre pilot study. Int J Probiotics Prebiotics 12(4) Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Borén T, Danielsson D, Teneberg S (2005) The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem 280:15390–15397 Urrutia-Baca VH, Escamilla-García E, de la Garza-Ramos MA, Tamez-Guerra P, Gomez-Flores R, Urbina-Ríos CS (2017) In vitro antimicrobial activity and downregulation of virulence gene expression on Helicobacter pylori by Reuterin. Probiotics Antimicrob Proteins 10(2):168–175 Vandenbergh PA (1993) Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev 12:221–238 Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T (2005) Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy 60:494–500 Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, JanC M, LaiC H, WangT N, Wang W (2004) Effects of ingesting Lactobacillus and Bifidobacterium containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr 80:737–741 Wang F, Feng J, Chen P, Liu X, Ma M, Zhou R, Chang Y, Liu J, Li J, Zhao Q (2017) Probiotics in Helicobacter pylori eradication therapy: systematic review and network meta-analysis. Res Hepatol Clin Gastroenterol 41:466–475 Wiese M, Eljaszewicz A, Andryszczyk M, Gronek S, Gackowska L, Kubiszewska I, Kaszewski W, Helmin-Basa A, Januszewska M, Motyl I, Wieczynska J, Michalkiewicz J (2012) Immunomodulatory effects of Lactobacillous plantarum and Helicobacter pylori CagA+ on the expression of selected superficial molecules on monocyte and lymphocyte and the synthesis of cytokines in whole blood culture. J Physiol Pharmacol 63(3):217–224 Wilhelm SM, Johnson JL, Kale-Pradhan PB (2011) Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother 45:960–966 Winter JA, Letley DP, Cook KW, Rhead JL, Zaitoun AA, Ingram RJ, Amilon KR, Croxall NJ, Kaye PV, Robinson K, Atherton JC (2014) A role for the vacuolating cytotoxin, VacA, in colonization and Helicobacter pylori-induced metaplasia in the stomach. J Infect Dis 210:954–963 Yang YJ, Chuang CC, Yang HB, Lu CC, Sheu BS (2012) Lactobacillus acidophilus ameliorates H. pylori-induced gastric inflammation by inactivating the Smad7 and NFκB pathways. BMC Microbiol 12:38 Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, Guo G, Zhao ZJ, Li L, Wu DL, Lu DS, Tan ZM, Liang HY, Wu C, Li DH, Luo P, Zeng H, Zhang WJ, Zhang JY, Guo BT, Zhu FC, Zou QM (2015) Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386:1457–1464 Zheng PY, Jones NL (2003) Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol 5:25–40 Zheng X, Lyu L, Mei Z (2013) Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig 105:445–453 Zhou C, Ma FZ, Deng XJ, Yuan H, Ma HS (2008) Lactobacilli inhibit interleukin-8 production induced by Helicobacter pylori lipopolysaccharide-activated toll-like receptor 4. World J Gastroenterol 14:5090–5095 Zhou Z, Gong S, Li XM, Yang Y, Guan R, Zhou S, Yao S, **e Y, Ou Z, Zhao J, Liu Z (2015) Expression of Helicobacter pylori urease B on the surface of Bacillus subtilis spores. J Med Microbiol 64(Pt):104–110References
Funding
This work was funded by the following organizations: The National Science Foundation of China (Grant numbers: 318755 and 316014489); International Science and Technology Cooperation Program of China (Grant number: 2013DFA32330); and National Science Foundation of Zhejiang Province (Grant number LY16C200002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qureshi, N., Li, P. & Gu, Q. Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen?. Appl Microbiol Biotechnol 103, 1573–1588 (2019). https://doi.org/10.1007/s00253-018-09580-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09580-3