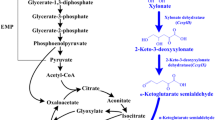
Abstract
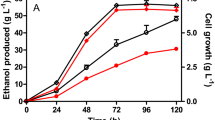
Escherichia coli KJ122 was previously engineered to produce high concentration and yield of succinate in mineral salt medium containing glucose and sucrose under anaerobic conditions. However, this strain does not efficiently utilize xylose. To improve the xylose uptake and utilization in the strain KJ122, xylFGH and xylE genes were individually and simultaneously deleted. E. coli KJ12201 (KJ122::ΔxylFGH) exhibited superior abilities in growth, xylose consumption, and succinate production compared to those of the parental strain KJ122. However, E. coli KJ12202 (KJ122::ΔxylE) lessened xylose consumption due to an ATP deficit for metabolizing xylose thus making succinate production from xylose not preferable. Moreover, E. coli KJ12203 (KJ122::ΔxylFGHΔxylE) exhibited an impaired growth on xylose due to lacking of xylose transporters. After performing metabolic evolution, the evolved KJ12201-14T strain exhibited a great improvement in succinate production from pure xylose with higher concentration and productivity about 18 and 21%, respectively, compared to KJ12201 strain. During fed-batch fermentation, KJ12201-14T also produced succinate from xylose at a concentration, yield, and overall productivity of 84.6 ± 0.7 g/L, 0.86 ± 0.01 g/g and 1.01 ± 0.01 g/L/h, respectively. KJ12201 and KJ12201-14T strains co-utilized glucose/xylose mixture without catabolite repression. Both strains produced succinate from glucose/xylose mixture at concentration, yield, and overall and specific productivities of about 85 g/L, 0.85 g/g, 0.70 g/L/h, and 0.44 g/gCDW/h, respectively. Based on our results, KJ12201 and KJ12201-14T strains exhibited a greater performance in succinate production from xylose containing medium than those of other published works. They would be potential strains for the economic bio-based succinate production from xylose.






Similar content being viewed by others
References
Aluri S, Rex K, Varshney U (2015) Simultaneous presence of fhs and purT genes is disadvantageous for the fitness of Escherichia coli growth. FEMS Microbiol Lett 362:1–6
Andersson C, Hodge D, Berglund KA, Rova U (2007) Effect of different carbon sources on the production of succinic acid using metabolically engineered Escherichia coli. Biotechnol Prog 23:381–388
Bao H, Liu R, Liang L, Jiang Y, Jiang M, Ma J, Chen K, Jia H, Wei P, Ouyang P (2014) Succinic acid production from hemicellulose hydrolysate by an Escherichia coli mutant obtained by atmospheric and room temperature plasma and adaptive evolution. Enzyme Microbiol Technol 66:10–15
Brink HG, Nicol W (2014) Succinic acid production with Actinobacillus succinogenes: rate and yield analysis of chemostat and biofilm cultures. Microb Cell Factories 13:111
Chundawat SPS, Beckham GT, Himmel ME, Dale BE (2011) Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol Eng 2:121–145
Chan S, Kanchanatawee S, Jantama K (2012) Production of succinic acid from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Bioresour Technol 103:329–336
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. PNAS 97:6640–6645
Delhomme C, Weuster-Botz D, Kuhn FE (2009) Succinic acid from renewable resources as a C4 building-block chemical—a review of the catalytic possibilities in aqueous media. Green Chem 11:13–26
Gonzalez R, Tao H, Shanmugam KT, York SW, Ingram LO (2002) Global gene expression differences associated with changes in glycolytic flux and growth rate in Escherichia coli during the fermentation of glucose and xylose. Biotechnol Prog 18:6–20
Grabar TB, Zhou S, Shanmugam KT, Yomano LP, Ingram LO (2006) Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(−) lactate fermentations by recombinant Escherichia coli. Biotechnol Lett 28:1527–1535
Hasona A, Kim Y, Healy F, Shanmugam KT, Ingram LO (2004) Pyruvate formate lyase and acetate kinase are essential for anaerobic growth of Escherichia coli on xylose. J Bacteriol 186:7593–7600
Hahn-Hagerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008a) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99:1140–1153
Jantama K, Zhang X, Moore JC, Svoronos SA, Shanmugam KT, Ingram LO (2008b) Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101:881–893
Jantama K, Polyiam P, Khunnonkwao P, Chan S, Sangproo M, Khor K, Jantama SS, Kanchanatawee S (2015) Efficient reduction of the formation of by-products and improvement of production yield of 2,3-butanediol by a combined deletion of alcohol dehydrogenase, acetate kinase-phosphotransacetylase, and lactate dehydrogenase genes in metabolically engineered Klebsiella oxytoca in mineral salts medium. Metab Eng 30:16–26
Jiang M, Wan Q, Liu R, Liang L, Chen X, Wu M, Zhang H, Chen K, Ma J, Wei P, Ouyang P (2014) Succinic acid production from corn stalk hydrolysate in an Escherichia coli mutant generated by atmospheric and room temperature plasmas and metabolic evolution strategies. J Ind Microbiol Biotechnol 41:115–123
Khor K, Sawisit A, Chan S, Kanchanatawee S, Jantama SS, Jantama K (2016) High production yield and specific productivity of succinate from cassava starch by metabolically-engineered Escherichia coli KJ122. J Chem Technol Biotechnol 91:2834–2841
Kwon YD, Kwon OH, Lee HS, Kim P (2007) The effect of NADP-dependent malic enzyme expression and anaerobic C4 metabolism in Escherichia coli compared with other anaplerotic enzymes. J Appl Microbiol 103:2340–2345
Lam VM, Daruwalla KR, Henderson PJ, Jones-Mortimer MC (1980) Proton-linked D-xylose transport in Escherichia coli. J Bacteriol 143:396–402
Liang L, Liu R, Li F, Wu M, Chen K, Ma J, Jiang M, Wei P, Ouyang P (2013) Repetitive succinic acid production from lignocellulose hydrolysates by enhancement of ATP supply in metabolically engineered Escherichia coli. Bioresour Technol 143:405–412
Lin H, Bennett GN, San KY (2005) Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol Bioeng 90:775–779
Lin SKC, Du C, Koutinas A, Wang R, Webb C (2008) Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem Eng J 41:128–135
Liu R, Liang L, Chen K, Ma J, Jiang M, Wei P, Ouyang P (2012) Fermentation of xylose to succinate by enhancement of ATP supply in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 94:959–968
Liu R, Liang L, Li F, Wu M, Chen K, Ma J, Jiang M, Wei P, Ouyang P (2013) Efficient succinic acid production from lignocellulosic biomass by simultaneous utilization of glucose and xylose in engineered Escherichia coli. Bioresour Technol 149:84–91
Martinez A, Grabar TB, Shanmugam KT, Yomano LP, York SW, Ingram LO (2007) Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol Lett 29:397–404
Marolewski A, Smith JM, Benkovic SJ (1994) Cloning and characterization of a new purine biosynthetic enzyme: a non-folate glycinamide ribonucleotide transformylase from E. coli. Biochemistry 33:2531–2537
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Posfai G, Koob MD, Kirkpatrick HA, Blattner FR (1997) Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol 179:4426–4428
Sawisit A, Jantama SS, Kanchanatawee S, Jantama K (2015a) Efficient utilization of cassava pulp for succinate production by metabolically engineered Escherichia coli KJ122. Bioprocess. Biosyst Eng 38:175–187
Sawisit A, Jantama K, Zheng H, Yomano LP, York SW, Shanmugam KT, Ingram LO (2015b) Mutation in galP improved fermentation of mixed sugars to succinate using engineered Escherichia coli AS1600a and AM1 mineral salts medium. Bioresour Technol 193:433–441
Sumiya M, Davis EO, Packman LC, McDonald TP, Henderson PJ (1995) Molecular genetics of a receptor protein for D-xylose, encoded by the gene xylF, in Escherichia coli. Receptors Channels 3:117–128
Sun L, Zeng X, Yan C, Sun X, Gong X, Rao Y, Yan N (2012) Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature 490:361–366
Utrilla J, Licona-Cassani C, Marcellin E, Gosset G, Nielsen LK, Martinez A (2012) Engineering and adaptive evolution of Escherichia coli for D(−) lactate fermentation reveals GatC as a xylose transporter. Metabolic Eng 14:469–476
Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72:3653–3661
Wang D, Li Q, Yang M, Zhang Y, Su Z, **ng J (2011) Efficient production of succinic acid from corn stalk hydrolysates by a recombinant Escherichia coli with ptsG mutation. Process Biochem 46:365–371
Wang X, Yomano LP, Lee JY, York SW, Zheng H, Mullinnix MT, Shanmugam KT, Ingram LO (2013) Engineering furfural tolerance in Escherichia coli improves the fermentation of lignocellulosic sugars into renewable chemicals. PNAS 110:4021–4026
Willke TH, Vorlop KD (2004) Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl Microbiol Biotechol 66:131–142
**a T, Altman E, Eiteman MA (2015) Succinate production from xylose-glucose mixtures using a consortium of engineered Escherichia coli. Eng Life Sci 15:65–72
Yomano LP, York SW, Zhou S, Shanmugam KT, Ingram LO (2008) Re-engineering Escherichia coli for ethanol production. Biotechnol Lett 30:2097–2103
Zhang X, Jantama K, Shanmugam KT, Ingram LO (2009) Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol 75:7807–7813
Zhang F, Li J, Liu H, Liang Q, Qi Q (2016) ATP-based ratio regulation of glucose and xylose improved succinate production. PLoS One 11:e0157775. https://doi.org/10.1371/journal. pone.0157775
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003) Production of optically pure D(−) lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhou S, Grabar TB, Shanmugam KT, Ingram LO (2006) Betaine tripled the volumetric productivity of D(−) lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett 28:671–676
Acknowledgements
We thank Prof. Dr. Lonnie Ingram, University of Florida, who cordially provided E. coli KJ122 used in this study. Thanks are also expressed to staff of the Office of International Relations at Ubon Ratchathani University for assistance with English.
Funding
This work was financially supported by the National Research Council of Thailand (NRCT). Miss Panwana also received the Excellent Graduate Student Fellowship at Suranaree University of Technology during performance of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 40 kb).
Rights and permissions
About this article
Cite this article
Khunnonkwao, P., Jantama, S.S., Kanchanatawee, S. et al. Re-engineering Escherichia coli KJ122 to enhance the utilization of xylose and xylose/glucose mixture for efficient succinate production in mineral salt medium. Appl Microbiol Biotechnol 102, 127–141 (2018). https://doi.org/10.1007/s00253-017-8580-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8580-2