Abstract
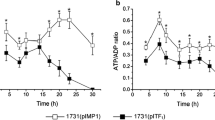
Clostridium beijerinckii is identified as a promising Clostridium strain for industrialization of acetone and butanol (AB) fermentation. It has been reported that high reducing power levels are associated with high butanol yield. In this study, we regulated reducing power by blocking NAD(P)H consumption in C. beijerinckii NCIMB 8052. Gene Cbei_4110, encoding NADH-quinone oxidoreductase (nuoG), is a subunit of the electron transport chain complex I. After inactivation of gene Cbei_4110, the generated mutant strain exhibited a remarkable increase in glucose utilization ratio and enhanced butanol production to 9.5 g/L in P2 medium containing 30 g/L of glucose. NAD(P)H and ATP levels were also increased by one to two times and three to five times, respectively. Furthermore, a comparative transcriptome analysis was carried out in order to determine the mechanism involved in the enhanced activity of the Cbei_4110-inactivated mutant strain. This strategy may be extended for making industrial bio-butanol more economically attractive.





Similar content being viewed by others
References
Abrahams JP, Leslie A, Lutter R, Walker JE (1994) Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature: 621–8.
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7(10):986–995
Baer SH, Hans PB, Smith TL (1987) Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl Environ Microb 53(12):2854–2861
Barabote RD, Saier MH (2005) Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol Mol Biol R 69(4):608–634
Bernofsky C, Swan M (1973) An improved cycling assay for nicotinamide adenine dinucleotide. Analytical Biochemistry. 53(2):452–458
Blair A, Ngo L, Park J, Paulsen IT, Saier MH (1996) Phylogenetic analyses of the homologous transmembrane channel-forming proteins of the F0F1-ATPases of bacteria, chloroplasts and mitochondria. Microbiology 142(1):17–32
Boyer PD (1993) The binding change mechanism for ATP synthase—some probabilities and possibilities. BBA-Bioenergetics 1140(3):215–250
Cooksley CM, Zhang Y, Wang H, Redl S, Winzer K, Minton NP (2012) Targeted mutagenesis of the Clostridium acetobutylicum acetone-butanol-ethanol fermentation pathway. Metab Eng 14:630–641
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotech 18(3):220–227
Gao H, Nielson F, Nielson HR (2011) Analysing protocol stacks for services. In: Wirsing M, Hölzl M (eds) Rigorous software engineering for service-oriented systems. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp. 369–389
Green EM (2011) Fermentative production of butanol-the industrial perspective. Curr Opin Biotech. 22(3):337–343
Grupe H, Gottschalk G (1992) Physiological events in Clostridium acetobutylicum during the shift from acidogenesis to solventogenesis in continuous culture and presentation of a model for shift induction. Appl Environ Microb 58(12):3896–3902
Guo T, Tang Y, Zhang QY, Du TF, Liang DF, Jiang M, Ouyang PK (2012) Clostridium beijerinckii mutant with high inhibitor tolerance obtained by low-energy ion implantation. J Ind Microbiol Biot 39(3):401–407
Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Meth 70:452–464
Heap JT, Pennington OJ, Cartman ST, Minton NP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Meth 78(1):79–85
Jang YS, Im JA, Choi SY, Lee JI, Lee SY (2014) Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab Eng 23:165–174
Jiang Y, Xu C, Dong F, Yang Y, Jiang W, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50(4):484
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36(suppl 1):D480–D484
Kuit W, Minton NP, López-Contreras AM, Eggink G (2012) Disruption of the acetate kinase (ack) gene of Clostridium acetobutylicum results in delayed acetate production. Appl Microbiol Biotechnol 94(3):729–741
Lee J, Blaschek HP (2001) Glucose uptake in Clostridium beijerinckii NCIMB 8052 and the solvent-hyperproducing mutant BA101. Appl Environ Microb 67(11):5025–5031
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101(2):209–228
Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J (2009) SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25(15):1966–1967
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiology Biot 69(6):627–642
Liu D, Chen Y, Ding F, Guo T, **e J, Zhuang W, Niu H, Shi X, Zhu C, Ying H (2015) Simultaneous production of butanol and acetoin by metabolically engineered Clostridium acetobutylicum. Metab Eng 27:107–114
Liu D, Chen Y, Li A, Ding F, Zhou T, He Y, Li B, Niu H, Lin X, **e J, Chen Y, Wu J, Ying H (2013) Enhanced butanol production by modulation of electron flow in Clostridium acetobutylicum B3 immobilized by surface adsorption. Bioresource Technol 129:321–328
Meyer CL, Papoutsakis ET (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30(5):450–459
Meyer CL, Roos JW, Papoutsakis ET (1986) Carbon monoxide gasing leads to alcohol production and butyrate uptake without acetone formation in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 24(2):159–167
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Map** and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Nicolet Y, Cavazza C, Fontecilla-Camps J (2002) Fe-only hydrogenases: structure, function and evolution. J Inorg Biochem 91(1):1–8
Nicolet Y, Lemon BJ, Fontecilla-Camps JC, Peters JW (2000) A novel FeS cluster in Fe-only hydrogenases. Trends Biochem Sci 25(3):138–143
Perutka J, Wang W, Goerlitz D, Lambowitz AM (2004) Use of computer-designed group II introns to disrupt Escherichia coli DExH/D-box protein and DNA helicase genes. J Mol Biol 336(2):421–439
Peters JW (1999) Structure and mechanism of iron-only hydrogenases. Curr Opin Struc Biol 9(6):670–676
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282(5395):1853–1858
Qureshi N, Ezeji TC, Ebener J, Dien BS, Cotta MA, Blaschek HP (2008) Butanol production by Clostridium beijerinckii. Part I: use of acid and enzyme hydrolyzed corn fiber. Bioresource Technol 99(13): 5915–5922.
Scheel M, Lutke-Eversloh T (2013) New options to engineer biofuel microbes: development and application of a high-throughput screening system. Metab Eng 17:51–58
Sillers R, Al-Hinai MA, Papoutsakis ET (2009) Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol Bioeng 102(1):38–49
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41(1):100–180
Tracy BP (2012) Improving butanol fermentation to enter the advanced biofuel market. mBio 3(6): e00518–512.
Ventura JR, Hu H, Jahng D (2013) Enhanced butanol production in Clostridium acetobutylicum ATCC 824 by double overexpression of 6-phosphofructokinase and pyruvate kinase genes. Appl Microbiol Biotechnol 97(16):7505–7516
Vignais PM (2008) Hydrogenases and H+-reduction in primary energy conservation. In: Schäfer G, Penefsky H (eds) Bioenergetics. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp. 223–252
Vignais PM, Billoud B, Meyer J (2001) Classification and phylogeny of hydrogenases. FEMS Microbiol Rev 25(4):455–501
Wang Y, Li X, Mao Y, Blaschek HP (2012) Genome-wide dynamic transcriptional profiling in Clostridium beijerinckii NCIMB 8052 using single-nucleotide resolution RNA-Seq. BMC Genomics 13(1):102
Wietzke M, Bahl H (2012) The redox-sensing protein Rex, a transcriptional regulator of solventogenesis in Clostridium acetobutylicum. Appl Microbiology Biot 96(3):749–761
**ao H, Gu Y, Ning Y, Yang Y, Mitchell WJ, Jiang W, Yang S (2011) Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl Environ Microb 77(22):7886–7895
**ao H, Li Z, Jiang Y, Yang Y, Jiang W, Gu Y, Yang S (2012) Metabolic engineering of D-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14:569–578
Yagi T (1993) The bacterial energy-transducing NADH-quinone oxidoreductases. BBA-Bioenergetics 1141(1):1–17
Yagi T, Yano T, Di Bernardo S, Matsuno-Yagi A (1998) Procaryotic complex I (NDH-1), an overview. BBA-Bioenergetics 1364(2):125–133
Zhao X, **ng D, Liu B, Lu L, Zhao J, Ren N (2012) The effects of metal ions and L-cysteine on hydA gene expression and hydrogen production by Clostridium beijerinckii RZF-1108. Int J Hydrog Energy 37(18):13711–13717
Acknowledgments
The authors would like to thank Prof. Nigel P. Minton and Michelle L. Kelly from the University of Nottingham, UK, for kindly providing the ClosTron plasmids and Prof. Sheng Yang from the Shanghai Institutes for Biological Sciences for kindly providing the pWJ1 plasmid.
This work was supported by the National Basic Research Program of China (973 Program, 2013CB733602), the restructured institutions innovation capacity of special funds of Ministry of Science and Technology of China (Grant No. 2014EG111227), the National Natural Science Foundation of China (Grant No. 21306032), the National High-Tech Research and Development Program of China (863) (Grant No. 2012AA021200), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Postdoctoral Science Foundation of Jiangsu Province (Grant No. 1302107C), the Major Research Plan of the National Natural Science Foundation of China (21390204), Program for Changjiang Scholars and Innovative Research Team in University (Grant No.: IRT_14R28), and Research Foundation of Guangzhou Sugarcane Industry Research Institute (Grant No. A201302).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This work is in compliance with ethical standards.
Conflict of Interest
The authors declare that they have no competing interests.
Human and animals studies
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Jun Liu, Ting Guo and Dong Wang contributed equally to this work.
Electronic supplementary materials
ESM 1
(PDF 286 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Guo, T., Wang, D. et al. Enhanced butanol production by increasing NADH and ATP levels in Clostridium beijerinckii NCIMB 8052 by insertional inactivation of Cbei_4110. Appl Microbiol Biotechnol 100, 4985–4996 (2016). https://doi.org/10.1007/s00253-016-7299-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7299-9