Abstract
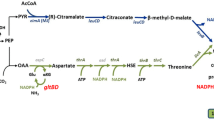
Systems metabolic engineering has made the renewable production of industrial chemicals a feasible alternative to modern operations. One major example of a renewable process is the production of carboxylic acids, such as octanoic acid (C8), from Escherichia coli, engineered to express thioesterase enzymes. C8, however, is toxic to E. coli above a certain concentration, which limits the final titer. 13C metabolic flux analysis of E. coli was performed for both C8 stress and control conditions using NMR2Flux with isotopomer balancing. A mixture of labeled and unlabeled glucose was used as the sole carbon source for bacterial growth for 13C flux analysis. By comparing the metabolic flux maps of the control condition and C8 stress condition, pathways that were altered under the stress condition were identified. C8 stress was found to reduce carbon flux in several pathways: the tricarboxylic acid (TCA) cycle, the CO2 production, and the pyruvate dehydrogenase pathway. Meanwhile, a few pathways became more active: the pyruvate oxidative pathway, and the extracellular acetate production. These results were statistically significant for three biological replicates between the control condition and C8 stress. As a working hypothesis, the following causes are proposed to be the main causes for growth inhibition and flux alteration for a cell under stress: membrane disruption, low activity of electron transport chain, and the activation of the pyruvate dehydrogenase regulator (PdhR).






Similar content being viewed by others
References
Alexeeva S, Hellingwerf KJ, de Mattos MJT (2002) Quantitative assessment of oxygen availability: perceived aerobiosis and its effect on flux distribution in the respiratory chain of Escherichia coli. J Bacteriol 184:1402–1406. doi:10.1128/JB.184.5.1402-1406.2002
Becker J, Klopprogge C, Herold A, Zelder O, Bolten CJ, Wittmann C (2007) Metabolic flux engineering of l-lysine production in Corynebacterium glutamicum—over expression and modification of G6P dehydrogenase. J Biotechnol 132:99–109. doi:10.1016/j.jbiotec.2007.05.026
Blank LM, Kuepfer L, Sauer U (2005) Large-scale C-13-flux analysis reveals mechanistic principles of metabolic network robustness to null mutations in yeast. Genome Biol 6.doi:10.1186/gb-2005-6-6-r49
Brynildsen MP, Liao JC (2009) An integrated network approach identifies the isobutanol response network of Escherichia coli. Mol Syst Biol 5.doi:10.1038/msb.2009.34
Choudhary MK, Gonzalez R, Yoon JM, Shanks JV (2011) Re-examination of metabolic fluxes in Escherichia coli during anaerobic fermentation of glucose using C-13 labeling experiments and 2-dimensional nuclear magnetic resonance (NMR) spectroscopy. Biotechnol Bioprocess Eng 16:419–437. doi:10.1007/s12257-010-0449-5
Desbois A, Smith V (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642. doi:10.1007/s00253-009-2355-3
Fischer E, Sauer U (2003) Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur J Biochem 270:880–891. doi:10.1046/j.1432-1033.2003.03448.x
Hager LP, Blake R, Gennis RB (1978) Activation of pyruvate oxidase by monomeric and micellar amphiphiles. J Biol Chem 253:1963–1971
Ingraham JL, Maaløe O, Neidhardt FC (1983) Growth of the bacterial cell. Sinauer Associates, Inc., Massachusetts
Jarboe LR, Royce LA, Liu P (2013) Understanding biocatalyst inhibition by carboxylic acids. Front Microbiol 4:272. doi:10.3389/fmicb.2013.00272
King T, Hinton JCD, Lucchini S, Gobius K (2010) Transcriptomic analysis of Escherichia coli o157:h7 and k-12 cultures exposed to inorganic and organic acids in stationary phase reveals acidulant- and strain-specific acid tolerance responses. Appl Environ Microbiol 76:6514–6528. doi:10.1128/AEM. 02392-09
Leighty RW, Antoniewicz MR (2012) Parallel labeling experiments with [U-C-13]glucose validate E. coli metabolic network model for C-13 metabolic flux analysis. Metab Eng 14:533–541. doi:10.1016/j.ymben.2012.06.003
Lennen RM, Kruziki MA, Kumar K, Zinkel RA, Burnum KE, Lipton MS, Hoover SW, Ranatunga DR, Wittkopp TM, Marner WD, Pfleger BF (2011) Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl Environ Microbiol 77:8114–28. doi:10.1128/AEM.05421-11
Li M, Agrawal A, Zhang X, San KY (2012) Effect of acetate formation pathway and long chain fatty acid CoA-ligase on the free fatty acid production in E. coli expressing acy-ACP thioesterase from Ricinus communis. Metab Eng 14:380–387. doi:10.1016/j.ymben.2012.03.007
Liu P, Najdi T, Chernyshov A, Dickerson J, Fu Y, Sandmeyer S, Jarboe L (2013) Membrane stress caused by octanoic acid in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 97:3239–3251. doi:10.1007/s00253-013-4773-5
Ogasawara H, Yamada K, Ishida Y, Yamamoto K, Ishihama A (2007) PdhR (pyruvate dehydrogenase complex regulator) controls the respiratory electron transport system in Escherichia coli. J Bacteriol 189:5534–5541. doi:10.1128/JB.00229-07
Ranganathan S, Tee TW, Chowdhury A, Zomorrodi AR, Yoon JM, Fu YF, Shanks JV, Maranas CD (2012) An integrated computational and experimental study for overproducing fatty acids in Escherichia coli. Metab Eng 14:687–704. doi:10.1016/j.ymben.2012.08.008
Royce L, Stebbins M, Liu P, Hanson B, Jarboe L (2013) The damaging effects of short chain fatty acids on Escherichia coli membranes. Appl Microbiol Biotechnol 97:8317–8327
Ruffing AM, Jones HDT (2012) Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol Bioeng 109:2190–2199. doi:10.1007/s00253-013-5113-5
Sauer U (2006) Metabolic networks in motion: C-13-based flux analysis. Mol Syst Biol 2. doi: 10.1038/msb4100109
Sauer U, Lasko DR, Fiaux J, Hochuli M, Glaser R, Szyperski T, Wuthrich KJ, Bailey E (1999) Metabolic flux ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. J Bacteriol 181:6679–6688
Schuetz R, Kuepfer L, Sauer U (2007) Systematic evaluation of objective functions for predicting intracellular fluxes in Escherichia coli. Mol Syst Biol 3. doi: 10.1038/msb4100162
Shanks BH (2010) Conversion of biorenewable feedstocks: new challenges in heterogeneous catalysis. Ind Eng Chem Res 49:10212–10217. doi:10.1021/ie100487r
Siddiquee KA, Arauzo-Bravo MJ, Shimizu K (2004) Metabolic flux analysis of pykF gene knockout Escherichia coli based on C-13-labeling experiments together with measurements of enzyme activities and intracellular metabolite concentrations. Appl Microbiol Biotechnol 63:407–417. doi:10.1007/s00253-003-1357-9
Sriram G, Fulton DB, Iyer VV, Peterson JM, Zhou R, Westgate ME, Spalding MH, Shanks JV (2006) Quantification of compartmented metabolic fluxes in develo** soybean embryos by employing biosynthetically directed fractional C-13 labeling, two-dimensional [C-13, H-1] nuclear magnetic resonance, and comprehensive isotopomer balancing. Plant Physiol 136:3043–3057. doi:10.1104/pp. 104.050625
Steen EJ, Kang YS, Bokinsky G, Hu ZH, Schirmer A, McClure A, del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–562. doi:10.1038/nature08721
Suthers PF, Burgard AP, Nowroozi F, Dasika MS, Van Dien S, Keasling JD, Maranas CD (2007) Metabolic flux elucidation for large-scale models using C-13 labeled isotopes. Metab Eng 9:387–405. doi:10.1016/j.ymben.2007.05.005
Szyperski T (1995) Biosynthetically directed fractional C-13 labeling of proteinogenic amino acids—an efficient analytical tool to investigate intermediary metabolism. Eur J Biochem 232:433–448. doi:10.1111/j.1432-1033.1995.433zz.x
Tee TW, Chowdhury A, Maranas CD, Shanks JV (2014) Systems metabolic engineering design: fatty acid production as an emerging case study. Biotechnol Bioeng 111:849–857. doi:10.1002/bit.25205
Toya Y, Ishii N, Nakahigashi K, Hirasawa T, Soga T, Tomita M, Shimizu K (2010) C-13 metabolic flux analysis for batch culture of Escherichia coli and its pyk and pgi gene knockout mutants based on mass isotopomer distribution of intracellular metabolites. Biotechnol Progr 26:975–992. doi:10.1002/btpr.420
van Winden W, Verheijen P, Schipper D, Heijnen J (2001) Innovations in generation and analysis of 2D [13C,1H] COSY NMR spectra for metabolic flux analysis purposes. Metab Eng 3:322–343. doi:10.1006/mben.2001.0193
Zamboni N, Ruhl M, Fendt SM, Sauer U (2009) C-13based metabolic flux analysis. Nat Protoc 4:878–892. doi:10.1038/nprot.2009.58
Zhang X, Agrawal A, Li M, San KY (2011) Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metab Eng 13:713–722. doi:10.1016/j.ymben.2011.09.007
Acknowledgments
The work is supported by the US National Science Foundation under Award Nos. EEC0813570 and BES-0331388/BES-0601549.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanfen Fu and Jong Moon Yoon equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 327 kb)
Rights and permissions
About this article
Cite this article
Fu, Y., Yoon, J.M., Jarboe, L. et al. Metabolic flux analysis of Escherichia coli MG1655 under octanoic acid (C8) stress. Appl Microbiol Biotechnol 99, 4397–4408 (2015). https://doi.org/10.1007/s00253-015-6387-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6387-6