Abstract
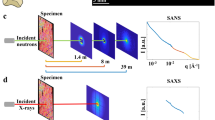
Despite the vast amount of studies focusing on bone nanostructure that have been performed for several decades, doubts regarding the detailed structure of the constituting hydroxyapatite crystal still exist. Different experimental techniques report somewhat different sizes and locations, possibly due to different requirements for the sample preparation. In this study, small- and wide-angle X-ray scattering is used to investigate the nanostructure of femur samples from young adult ovine, bovine, porcine, and murine cortical bone, including three different orthogonal directions relative to the long axis of the bone. The radially averaged scattering from all samples reveals a remarkable similarity in the entire q range, which indicates that the nanostructure is essentially the same in all species. Small differences in the data from different directions confirm that the crystals are elongated in the [001] direction and that this direction is parallel to the long axis of the bone. A model consisting of thin plates is successfully employed to describe the scattering and extract the plate thicknesses, which are found to be in the range of 20–40 Å for most samples but 40–60 Å for the cow samples. It is demonstrated that the mineral plates have a large degree of polydispersity in plate thickness. Additionally, and equally importantly, the scattering data and the model are critically evaluated in terms of model uncertainties and overall information content.








Similar content being viewed by others
References
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10(9):3815–3826. doi:10.1016/j.actbio.2014.05.024
Fratzl P, Gupta HS, Paschalis EP, Roschger P (2004) Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 14(14):2115–2123. doi:10.1039/b402005g
Stock SR (2015) The mineral-collagen interface in bone. Calcif Tissue Int. doi:10.1007/s00223-015-9984-6
Hodge AJ, Petruska JA (1963) Recent studies with the electron microscope on ordered aggregates of the tropocollagen molecule. In: Ramachandran GN (ed) Aspects of protein structure. Academic press, London and New York, pp 289–300
Landis WJ, Jacquet R (2013) Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif Tissue Int 93(4):329–337. doi:10.1007/s00223-013-9725-7
Weiner S, Arad T, Traub W (1991) Crystal organization in rat bone lamellae. FEBS Lett 285(1):49–54. doi:10.1016/0014-5793(91)80722-F
Landis WJ, Hodgens KJ, Song MJ, Arena J, Kiyonaga S, Marko M, Owen C, McEwen BF (1996) Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol 117(1):24–35. doi:10.1006/jsbi.1996.0066
Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB (2007) Bone structure and formation: a new perspective. Mater Sci Eng 58(3–5):77–116. doi:10.1016/j.mser.2007.05.001
Chen PY, Toroian D, Price PA, McKittrick J (2011) Minerals form a continuum phase in mature cancellous bone. Calcif Tissue Int 88(5):351–361. doi:10.1007/s00223-011-9462-8
Weiner S, Price PA (1986) Disaggregation of bone into crystals. Calcif Tissue Int 39(6):365–375. doi:10.1007/Bf02555173
McNally E, Nan F, Botton GA, Schwarcz HP (2013) Scanning transmission electron microscopic tomography of cortical bone using Z-contrast imaging. Micron 49:46–53. doi:10.1016/j.micron.2013.03.002
McNally EA, Schwarcz HP, Botton GA, Arsenault AL (2012) A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS One. doi:10.1371/journal.pone.0029258
Schwarcz HP, McNally EA, Botton GA (2014) Dark-field transmission electron microscopy of cortical bone reveals details of extrafibrillar crystals. J Struct Biol 188(3):240–248. doi:10.1016/j.jsb.2014.10.005
Fujisawa R, Kuboki Y (1991) Preferential adsorption of dentin and bone acidic proteins on the (100) face of hydroxyapatite crystals. Biochim Biophys Acta 1:56–60
Wang ZQ, Xu ZJ, Zhao WL, Sahai N (2015) A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J Mater Chem B 3(47):9157–9167. doi:10.1039/c5tb01036e
Hu YY, Rawal A, Schmidt-Rohr K (2010) Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci USA 107(52):22425–22429. doi:10.1073/pnas.1009219107
Handschin RG, Stern WB (1995) X-ray diffraction studies on the lattice perfection of human bone apatite (Crista Iliaca). Bone. doi:10.1016/S8756-3282(95)80385-8
Handschin RG, Stern WB (1992) Crystallographic lattice refinement of human bone. Calcif Tissue Int 51(2):111–120. doi:10.1007/Bf00298498
Trebacz H, Pikus S (2003) A study of mineral phase in immobilized rat femur: structure refinements by Rietveld analysis. J Bone Miner Metab 21(2):80–85. doi:10.1007/s007740300013
Selvig KA (1970) Periodic lattice images of hydroxyapatite crystals in human bone and dental hard tissues. Calcif Tiss Res 6(3):227–238. doi:10.1007/Bf02196203
Schmidt WI (1936) Über die Orientierung der Kristallite im Zahnschmel. Naturwissenschaften 24(23):361. doi:10.1007/BF01473678
Tadano S, Giri B (2011) X-ray diffraction as a promising tool to characterize bone nanocomposites. Sci Technol Adv Mater. doi:10.1088/1468-6996/12/6/064708
Meneghini C, Dalconi MC, Nuzzo S, Mobilio S, Wenk RH (2003) Rietveld refinement on X-ray diffraction patterns of bioapatite in human fetal bones. Biophys J 84(3):2021–2029. doi:10.1016/S0006-3495(03)75010-3
Smith CB, Smith DA (1978) Structural role of bone apatite in human femoral compacta. Acta Orthop Scand 49(5):440–444. doi:10.3109/17453677808993259
Wang Y, Von Euw S, Fernandes FM, Cassaignon S, Selmane M, Laurent G, Pehau-Arnaudet G, Coelho C, Bonhomme-Coury L, Giraud-Guille M-M, Babonneau F, Azaïs T, Nassif N (2013) Water-mediated structuring of bone apatite. Nat Mater 12(12):1144–1153. doi:10.1038/nmat3787
Jager C, Welzel T, Meyer-Zaika W, Epple M (2006) A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn Reson Chem 44(6):573–580. doi:10.1002/Mrc.1774
Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MMJ, Beck LW (2005) Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J Bone Miner Res 20(4):625–634. doi:10.1359/JBMR.041217
Weiner S, Traub W (1992) Bone-structure—from Angstroms to Microns. Faseb Journal 6(3):879–885
Boskey A (2003) Bone mineral crystal size. Osteoporos Int 14:S16–S20. doi:10.1007/s00198-003-1468-2
Fratzl P, Groschner M, Vogl G, Plenk H, Eschberger J, Fratzlzelman N, Koller K, Klaushofer K (1992) Mineral crystals in calcified tissues—a comparative-study by saxs. J Bone Miner Res 7(3):329–334
Fratzl P, Schreiber S, Klaushofer K (1996) Bone mineralization as studied by small-angle X-ray scattering. Connect Tissue Res 35(1–4):9–16
Fratzl P, Fratzlzelman N, Klaushofer K, Vogl G, Koller K (1991) Nucleation and growth of mineral crystals in bone studied by small-angle X-ray-scattering. Calcif Tissue Int 48(6):407–413. doi:10.1007/Bf02556454
Hiller JC, Wess TJ (2006) The use of small-angle X-ray scattering to study archaeological and experimentally altered bone. J Archaeol Sci 33(4):560–572. doi:10.1016/j.jas.2005.09.012
Bunger MH, Oxlund H, Hansen TK, Sorensen S, Bibby BM, Thomsen JS, Langdahl BL, Besenbacher F, Pedersen JS, Birkedal H (2010) Strontium and bone nanostructure in normal and ovariectomized rats investigated by scanning small-angle X-ray scattering. Calcif Tissue Int 86(4):294–306. doi:10.1007/s00223-010-9341-8
Fratzl P, Gupta HS, Paris O, Valenta A, Roschger P, Klaushofer K (2005) Diffracting “stacks of cards”—some thoughts about small-angle scattering from bone. In: Kremer F, Richtering W (eds) Scattering methods and the properties of polymer materials, vol 130., Progress in colloid and polymer scienceSpringer, Berlin, pp 33–39
Acerbo AS, Kwaczala AT, Yang L, Judex S, Miller LM (2014) Alterations in collagen and mineral nanostructure observed in osteoporosis and pharmaceutical treatments using simultaneous small- and wide-angle X-ray scattering. Calcif Tissue Int 95(5):446–456. doi:10.1007/s00223-014-9913-0
Georgiadis M, Guizar-Sicairos M, Zwahlen A, Trussel AJ, Bunk O, Muller R, Schneider P (2015) 3D scanning SAXS: a novel method for the assessment of bone ultrastructure orientation. Bone 71:42–52. doi:10.1016/j.bone.2014.10.002
Burger C, Zhou HW, Wang H, Sics I, Hsiao BS, Chu B, Graham L, Glimcher MJ (2008) Lateral packing of mineral crystals in bone collagen fibrils. Biophys J 95(4):1985–1992. doi:10.1529/biophysj.107.128355
Lindner P, Zemb T (2002) Neutrons, X-rays, and light: scattering methods applied to soft condensed matter, 1st edn., North-Holland delta series, Elsevier, Amsterdam, Boston
Shimada T, Doi M, Okano K (1988) Concentration fluctuation of stiff polymers. 1. Static structure factor. J Chem Phys 88(4):2815–2821. doi:10.1063/1.454016
Teixeira J (1988) Small-angle scattering by fractal systems. J Appl Crystallogr 21:781–785. doi:10.1107/s0021889888000263
Wagermaier W, Gupta HS, Gourrier A, Burghammer M, Roschger P, Fratzl P (2006) Spiral twisting of fiber orientation inside bone lamellae. Biointerphases 1(1):1–5. doi:10.1116/1.2178386
Schweizer KS, Curro JG (1994) Prism theory of the structure, thermodynamics, and phase-transitions of polymer liquids and alloys. Adv Polym Sci 116:319–377. doi:10.1007/Bfb0080203
Hughes JM, Cameron M, Crowley KD (1989) Structural variations in natural F, OH, and Cl apatites. Am Miner 74(7–8):870–876
Svergun D, Barberato C, Koch MHJ (1995) CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Crystallogr 28:768–773
Døvling Kaspersen J, Moestrup Jessen C, Stougaard Vad B, Skipper Sørensen E, Kleiner Andersen K, Glasius M, Pinto Oliveira CL, Otzen DE, Pedersen JS (2014) Low-Resolution structures of OmpA·DDM protein-detergent complexes. ChemBioChem 15(14):2113–2124. doi:10.1002/cbic.201402162
Pedersen JS (1999) Structural studies by small-angle scattering and specular reflectivity. Riso National Lab, Roskilde
Traub W, Arad T, Weiner S (1989) 3-Dimensional ordered distribution of crystals in Turkey tendon collagen-fibers. Proc Natl Acad Sci USA 86(24):9822–9826. doi:10.1073/pnas.86.24.9822
Pedersen JS, Vysckocil P, Schonfeld B, Kostorz G (1997) Small-angle neutron scattering of precipitates in Ni-rich Ni-Ti alloys. II. Methods for analyzing anisotropic scattering data. J Appl Crystallogr 30:975–984
Kotlarchyk M, Chen SH (1983) Analysis of small-angle neutron-scattering spectra from polydisperse interacting colloids. J Chem Phys 79(5):2461–2469
Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK (2004) High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35(1):4–10. doi:10.1016/j.bone.2004.02.024
Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ (2003) Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif Tissue Int 72(5):592–598. doi:10.1007/s00223-002-1077-7
Eppell SJ, Tong WD, Katz JL, Kuhn L, Glimcher MJ (2001) Shape and size of isolated bone mineralites measured using atomic force microscopy. J Orthop Res 19(6):1027–1034. doi:10.1016/S0736-0266(01)00034-1
Turunen MJ, Lages S, Labrador A, Olsson U, Tagil M, Jurvelin JS, Isaksson H (2014) Evaluation of composition and mineral structure of callus tissue in rat femoral fracture. J Biomed Opt. doi:10.1117/1.Jbo.19.2.025003
Acknowledgments
Funding from the Swedish Foundation for Strategic Research and the European Commission (FRACQUAL-293434) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any conflict of interest.
Human and Animal Rights and Informed Consent
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Kaspersen, J.D., Turunen, M.J., Mathavan, N. et al. Small-Angle X-ray Scattering Demonstrates Similar Nanostructure in Cortical Bone from Young Adult Animals of Different Species. Calcif Tissue Int 99, 76–87 (2016). https://doi.org/10.1007/s00223-016-0120-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0120-z