Abstract
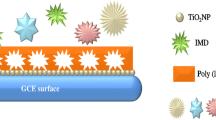
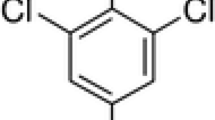
In the present study, a peptide nanotube functionalized polydopamine (p-Dop) based molecularly imprinted (MIP) sensor system was constructed, characterized, and studied for the impedimetric sensing of an organophosphorus pesticide, malathion (MLT). Electropolymerization in the presence of a template (MLT) was utilized as a convenient and effective strategy to generate imprinted p-Dop films on peptide nanotubes (PNTs) modified graphite electrodes (PGEs). Upon the removal of template, the adsorption of MLT on the specific cavities formed in the MIP film was tracked using electrochemical impedance spectroscopy (EIS). To attain optimal sensor response, experimental conditions, such as film thickness, analyte/functional monomer ratio, and desorption/adsorption time, were analyzed. The obtained MIP(p-Dop)-PNT-PGE sensor exhibited high sensitivity for electrochemical MLT analysis with a wide dynamic detection range of 13 pg mL−1 – 1.3 µg mL−1 and a LOD of 1.39 pg mL−1. The combination of a bio-inspired p-Dop-based MIP with the EIS technique allowed excellent sensitivity and selectivity toward MLT sensing which also yielded high recoveries in real samples. The success of this research strategy in real samples revealed its potential for various future environmental applications.
Graphical abstract










Similar content being viewed by others
References
Rebelo P, Costa-Rama E, Seguro I, Pacheco JG, Nouws HPA, Cordeiro MNDS, Delerue-Matos C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens Bioelectron. 2021;172:112719.
Liu H, Liu Z, Yi J, Ma D, **a F, Tian D, Zhou C. A dual-signal electroluminescence aptasensor based on hollow Cu/Co-MOF-luminol and g-C3N4 for simultaneous detection of acetamiprid and malathion. Sensors Actuators B Chem. 2021;331:129412. https://doi.org/10.1016/j.snb.2020.129412.
Xu G, Huo D, Hou J, Zhang C, Zhao Y, Hou C, Bao J, Yao X, Yang M. An electrochemical aptasensor of malathion based on ferrocene/DNA-hybridized MOF, DNA coupling-gold nanoparticles and competitive DNA strand reaction. Microchem J. 2021;162:105829. https://doi.org/10.1016/j.microc.2020.105829.
Kaur N, Thakur H, Prabhakar N. Multi walled carbon nanotubes embedded conducting polymer based electrochemical aptasensor for estimation of malathion. Microchem J. 2019;147:393–402. https://doi.org/10.1016/j.microc.2019.03.042.
**ao Z, He M, Chen B, Hu B. Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to gas chromatography-flame photometric detection for the determination of organophosphorus pesticides in environmental water samples. Talanta. 2016;156–157:126–33. https://doi.org/10.1016/j.talanta.2016.05.001.
Albuquerque CDL, Poppi RJ. Detection of malathion in food peels by surface-enhanced Raman imaging spectroscopy and multivariate curve resolution. Anal Chim Acta. 2015;879:24–33. https://doi.org/10.1016/j.aca.2015.04.019.
Tang T, Deng J, Zhang M, Shi G, Zhou T. Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: a universal strategy for ratiometric detection of organophosphorus pesticides. Talanta. 2016;146:55–61. https://doi.org/10.1016/j.talanta.2015.08.023.
Yao J, Wang Z, Guo L, Xu X, Liu L, Xu L, Song S, Xu C, Kuang H. Advances in immunoassays for organophosphorus and pyrethroid pesticides. TrAC Trends Anal Chem. 2020;131:116022. https://doi.org/10.1016/j.trac.2020.116022.
Chen H, Hu O, Fan Y, Xu L, Zhang L, Lan W, Hu Y, **e X, Ma L, She Y, Fu H. Fluorescence paper-based sensor for visual detection of carbamate pesticides in food based on CdTe quantum dot and nano ZnTPyP. Food Chem. 2020;327. https://doi.org/10.1016/j.foodchem.2020.127075
Xu G, Hou J, Zhao Y, Bao J, Yang M, Fa H, Yang Y, Li L, Huo D, Hou C. Dual-signal aptamer sensor based on polydopamine-gold nanoparticles and exonuclease I for ultrasensitive malathion detection. Sensors Actuators B Chem. 2019;287:428–36. https://doi.org/10.1016/j.snb.2019.01.113.
Guler M, Turkoglu V, Kivrak A. Electrochemical detection of malathion pesticide using acetylcholinesterase biosensor based on glassy carbon electrode modified with conducting polymer film. Environ Sci Pollut Res. 2016;23:12343–51. https://doi.org/10.1007/s11356-016-6385-y.
Rhouati A, Majdinasab M, Hayat A. A perspective on non-enzymatic electrochemical nanosensors for direct detection of pesticides. Curr Opin Electrochem. 2018;11:12–8. https://doi.org/10.1016/j.coelec.2018.06.013.
**e Y, Yu Y, Lu L, Ma X, Gong L, Huang X, Liu G, Yu Y. CuO nanoparticles decorated 3D graphene nanocomposite as non-enzymatic electrochemical sensing platform for malathion detection. J Electroanal Chem. 2018;812:82–9. https://doi.org/10.1016/j.jelechem.2018.01.043.
Wang W, Wang X, Cheng N, Luo Y, Lin Y, Xu W, Du D. Recent advances in nanomaterials-based electrochemical (bio)sensors for pesticides detection. TrAC Trends Anal Chem. 2020;132:116041. https://doi.org/10.1016/j.trac.2020.116041.
Belbruno JJ. Molecularly ımprinted polymers. Chem Rev. 2019;119:94–119.
Jia M, Zhang Z, Li J, Ma X, Chen L, Yang X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal Chem. 2018;106:190–201. https://doi.org/10.1016/j.trac.2018.07.011.
Jesadabundit W, Jampasa S, Patarakul K, Siangproh W, Chailapakul O. Enzyme-free impedimetric biosensor-based molecularly imprinted polymer for selective determination of L-hydroxyproline. Biosens Bioelectron. 2021;191:113387. https://doi.org/10.1016/j.bios.2021.113387.
Aghoutane Y, Diouf A, Österlund L, Bouchikhi B, El Bari N. Development of a molecularly imprinted polymer electrochemical sensor and its application for sensitive detection and determination of malathion in olive fruits and oils. Bioelectrochemistry. 2020;132:107404. https://doi.org/10.1016/j.bioelechem.2019.107404.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30. https://doi.org/10.1126/science.1147241.
Jiang J, Zhu L, Zhu L, Zhu B, Xu Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir. 2011;27:14180–7. https://doi.org/10.1021/la202877k.
He H, **e Q, Yao S. An electrochemical quartz crystal impedance study on anti-human immunoglobulin G immobilization in the polymer grown during dopamine oxidation at an Au electrode. J Colloid Interface Sci. 2005;289:446–54. https://doi.org/10.1016/j.jcis.2005.03.085.
Liu K, Wei WZ, Zeng JX, Liu XY, Gao YP. Application of a novel electrosynthesized polydopamine-imprinted film to the capacitive sensing of nicotine. Anal Bioanal Chem. 2006;385:724–9. https://doi.org/10.1007/s00216-006-0489-z.
Zhou WH, Tang SF, Yao QH, Chen FR, Yang HH, Wang XR. A quartz crystal microbalance sensor based on mussel-inspired molecularly imprinted polymer. Biosens Bioelectron. 2010;26:585–9. https://doi.org/10.1016/j.bios.2010.07.024.
Yang B, Lv S, Chen F, Liu C, Cai C, Chen C, Chen X. A resonance light scattering sensor based on bioinspired molecularly imprinted polymers for selective detection of papain at trace levels. Anal Chim Acta. 2016;912:125–32. https://doi.org/10.1016/j.aca.2016.01.030.
Lan F, Ma S, Yang Q, **e L, Wu Y, Gu Z. Polydopamine-based superparamagnetic molecularly imprinted polymer nanospheres for efficient protein recognition. Colloids Surfaces B Biointerfaces. 2014;123:213–8. https://doi.org/10.1016/j.colsurfb.2014.09.018.
Miao J, Liu A, Wu L, Yu M, Wei W, Liu S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal Chim Acta. 2020;1095:82–92. https://doi.org/10.1016/j.aca.2019.10.027.
Yang YY, Li YT, Li XJ, Zhang L, Kouadio Fodjo E, Han S. Controllable in situ fabrication of portable AuNP/mussel-inspired polydopamine molecularly imprinted SERS substrate for selective enrichment and recognition of phthalate plasticizers. Chem Eng J. 2020;402:125179. https://doi.org/10.1016/j.cej.2020.125179.
Turco A, Corvaglia S, Mazzotta E, Pompa PP, Malitesta C. Preparation and characterization of molecularly imprinted mussel inspired film as antifouling and selective layer for electrochemical detection of sulfamethoxazole. Sensors Actuators B Chem. 2018;255:3374–83. https://doi.org/10.1016/j.snb.2017.09.164.
Yin ZZ, Cheng SW, Bin XuL, Liu HY, Huang K, Li L, Zhai YY, Zeng YB, Liu HQ, Shao Y, Zhang ZL, Lu YX. Highly sensitive and selective sensor for sunset yellow based on molecularly imprinted polydopamine-coated multi-walled carbon nanotubes. Biosens Bioelectron. 2018;100:565–70. https://doi.org/10.1016/j.bios.2017.10.010.
Radi AE, Eissa A, Wahdan T. Impedimetric sensor for deoxynivalenol based on electropolymerised molecularly imprinted polymer on the surface of screen-printed gold electrode. Int J Environ Anal Chem. 2019;00:1–12. https://doi.org/10.1080/03067319.2019.1699548.
Shamsipur M, Moradi N, Pashabadi A. Coupled electrochemical-chemical procedure used in construction of molecularly imprinted polymer-based electrode: a highly sensitive impedimetric melamine sensor. J Solid State Electrochem. 2018;22:169–80. https://doi.org/10.1007/s10008-017-3731-z.
Bolat G, Yaman YT, Abaci S. Molecularly imprinted electrochemical impedance sensor for sensitive dibutyl phthalate (DBP) determination. Sensors Actuators B Chem. 2019;299:127000. https://doi.org/10.1016/j.snb.2019.127000.
Karami P, Bagheri H, Johari-Ahar M, Khoshsafar H, Arduini F, Afkhami A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta. 2019;202:111–22. https://doi.org/10.1016/j.talanta.2019.04.061.
Yaman YT, Bolat G, Saygin TB, Abaci S. Molecularly imprinted label-free sensor platform for impedimetric detection of 3-monochloropropane-1,2˗diol. Sensors Actuators B Chem. 2021;328:128986. https://doi.org/10.1016/j.snb.2020.128986.
Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625 LP – 627.
Sedman VL, Adler-Abramovich L, Allen S, Gazit E, Tendler SJB. Direct observation of the release of phenylalanine from diphenylalanine nanotubes. J Am Chem Soc. 2006;128:6903–8. https://doi.org/10.1021/ja060358g.
Adler-abramovich L, Reches M, Sedman VL, Allen S, Tendler SJB, Gazit E, Uni T, Ng N. Thermal and chemical stability of diphenylalanine peptide nanotubes : ımplications for nanotechnological applications. Langmuir. 2006;22:1313–20. https://doi.org/10.1021/la052409d.
Zhao L, Zou Q, Yan X. Self-assembling peptide-based nanoarchitectonics. Bull Chem Soc Jpn. 2019;92:70–9. https://doi.org/10.1246/bcsj.20180248.
Levin A, Hakala TA, Schnaider L, Bernardes GJL, Gazit E, Knowles TPJ. Biomimetic peptide self-assembly for functional materials. Nat Rev Chem. 2020;4:615–34. https://doi.org/10.1038/s41570-020-0215-y.
Yuan C, Ji W, **ng R, Li J, Gazit E, Yan X. Hierarchically oriented organization in supramolecular peptide crystals. Nat Rev Chem. 2019;3:567–88. https://doi.org/10.1038/s41570-019-0129-8.
Yemini M, Reches M, Gazit E, Rishpon J. Peptide nanotube-modified electrodes for enzyme-biosensor applications. Anal Chem. 2005;77:5155–9. https://doi.org/10.1021/ac050414g.
Adler-Abramovich L, Badihi-Mossberg M, Gazit E, Rishpon J. Characterization of peptide-nanostructure-modified electrodes and their application for ultrasensitive environmental monitoring. Small. 2010;6:825–31. https://doi.org/10.1002/smll.200902186.
De La Rica R, Mendoza E, Lechuga LM, Matsui H. Label-free pathogen detection with sensor chips assembled from peptide nanotubes. Angew Chem Int Ed. 2008;47:9752–5. https://doi.org/10.1002/anie.200804299.
Castillo JJ, Svendsen WE, Rozlosnik N, Escobar P, Martínez F, Castillo-León J. Detection of cancer cells using a peptide nanotube-folic acid modified graphene electrode. Analyst. 2013;138:1026–31. https://doi.org/10.1039/c2an36121c.
Bolat G, Abaci S, Vural T, Bozdogan B, Denkbas EB. Sensitive electrochemical detection of fenitrothion pesticide based on self-assembled peptide-nanotubes modified disposable pencil graphite electrode. J Electroanal Chem. 2018;809:88–95. https://doi.org/10.1016/j.jelechem.2017.12.060.
Leibl N, Duma L, Gonzato C, Haupt K. Polydopamine-based molecularly imprinted thin films for electro-chemical sensing of nitro-explosives in aqueous solutions. Bioelectrochemistry. 2020;135:107541. https://doi.org/10.1016/j.bioelechem.2020.107541.
Stöckle B, Ng DYW, Meier C, Paust T, Bischoff F, Diemant T, Behm RJ, Gottschalk KE, Ziener U, Weil T. Precise control of polydopamine film formation by electropolymerization. Macromol Symp. 2014;346:73–81. https://doi.org/10.1002/masy.201400130.
Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW. Perspectives on poly(dopamine). Chem Sci. 2013;4:3796–802. https://doi.org/10.1039/c3sc51501j.
Ding YH, Floren M, Tan W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribol. 2016;2:121–36. https://doi.org/10.1016/j.bsbt.2016.11.001.
Yemini M, Reches M, Gazit E, Rishpon J, Aviv T. Peptide nanotube-modified electrodes for enzyme - biosensor applications. Anal Chem. 2005;77:5155–9.
Chan E, Choi J, Lee M, Koo K. Fabrication of an electrochemical immunosensor with self-assembled peptide nanotubes. Colloids Surf A Physicochem Eng Asp. 2008;314:95–9. https://doi.org/10.1016/j.colsurfa.2007.04.154.
Yemini M, Reches M, Rishpon J, Gazit E. Novel electrochemical biosensing platform using self-assembled peptide nanotubes. Nano Lett. 2005;5:183–6.
Laureana L, Pompa PP, Maruccio G, Della TA, Sabella S, Tamburro AM, Cingolani R, Rinaldi R. Charge transport and intrinsic fluorescence in amyloid-like fibrils. PNAS. 2007;104:18019–24.
Ashkenasy N, Horne WS, Ghadiri MR. Design of self-assembling peptide nanotubes with delocalized electronic states**. Small. 2006;2:99–102. https://doi.org/10.1002/smll.200500252.
Quintás G, Garrigues S, De GM. FT –Raman spectrometry determination of malathion in pesticide formulations. Talanta. 2004;63:345–50. https://doi.org/10.1016/j.talanta.2003.11.004.
Sharma AK, Tiwari U, Gaur MS, Tiwari RK. Assessment of malathion and its effects on leukocytes in human blood samples. J Biomed Res. 2016;30:52–9. https://doi.org/10.7555/JBR.30.20120073.
Mirković MM, Pašti TDL, Došen AM, Čebela MŽ, Rosić AA, Matović BZ, Babić BM. Adsorption of malathion on mesoporous monetite obtained by mechanochemical treatment of brushite. RSC Adv. 2016;6:12219–25. https://doi.org/10.1039/C5RA27554G.
Pandey GP, Singh AK, Deshmukh L, Prasad S, Paliwal LJ, Asthana A, Mathew SB. A novel and sensitive kinetic method for the determination of malathion using chromogenic reagent. Microchem J. 2014;113:83–9. https://doi.org/10.1016/j.microc.2013.11.005.
Donia AM, Atia AA, Hussien RA, Rashad RT. Comparative study on the adsorption of malathion pesticide by different adsorbents from aqueous solution. Desalin Water Treat. 2012;47:300–9. https://doi.org/10.1080/19443994.2012.696419.
Chatterjee S, Das SK, Chakravarty R, Chakrabarti A, Ghosh S, Guha AK. Interaction of malathion, an organophosphorus pesticide with Rhizopus oryzae biomass. J Hazard Mater. 2010;174:47–53. https://doi.org/10.1016/j.jhazmat.2009.09.014.
Hermosillo-nevárez JJ, Bustos-terrones V, Bustos-terrones YA, Uriarte-Aceves PM, Rangel-Peraza JG. Feasibility study on the use of recycled polymers for malathion adsorption: ısotherms and kinetic modeling. Materials (Basel). 2020;13:1824. https://doi.org/10.3390/ma13081824.
Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12:8882–92. https://doi.org/10.1021/acsnano.8b04022.
Yu X, Fan H, Liu Y, Shi Z, ** Z. Characterization of carbonized polydopamine nanoparticles suggests ordered supramolecular structure of polydopamine. Langmuir. 2014;30:5497–505. https://doi.org/10.1021/la500225v.
Luo H, Gu C, Zheng W, Dai F, Wang X, Zheng Z. Facile synthesis of novel size-controlled antibacterial hybrid sphere with silver nanoparticles loaded to poly-dopamine sphere. RSC Adv. 2015;5:13470–7. https://doi.org/10.1039/b000000x.
Tamanna T, Yu A. Polydopamine nanoparticle as a stable and capacious nano-reservoir of Rifampicin. Int J Pharmacol Pharm Sci. 2016;10:56–9. https://doi.org/10.1017/CBO9781107415324.004.
Bolat G, Abaci S. Non-enzymatic electrochemical sensing of malathion pesticide in tomato and apple samples based on gold nanoparticles-chitosan-ionic liquid hybrid nanocomposite. Sensors (Switzerland). 2018;18. https://doi.org/10.3390/s18030773.
Liang AA, Hou BH, Tang CS, Sun DL, Luo EA. Bioelectrochemistry An advanced molecularly imprinted electrochemical sensor for the highly sensitive and selective detection and determination of Human IgG. Bioelectrochemistry. 2021;137:107671. https://doi.org/10.1016/j.bioelechem.2020.107671.
Khumsap T, Bamrungsap S, Thu VT, Nguyen LT. Epitope-imprinted polydopamine electrochemical sensor for ovalbumin detection. Bioelectrochemistry. 2021;140:107805. https://doi.org/10.1016/j.bioelechem.2021.107805.
Guo W, Pi F, Zhang H, Sun J, Zhang Y, Sun X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens Bioelectron. 2017;98:299–304. https://doi.org/10.1016/j.bios.2017.06.036.
Zuo HG, Zhu JX, Zhan CR, Shi L, **ng M, Guo P, Ding Y, Yang H. Preparation of malathion MIP-SPE and its application in environmental analysis. Environ Monit Assess. 2015;187. https://doi.org/10.1007/s10661-015-4641-0.
Funding
The authors received the financial support of this work under ID number FBA-2019–18385 by Research Council of Hacettepe University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yaman, Y.T., Bolat, G., Abaci, S. et al. Peptide nanotube functionalized molecularly imprinted polydopamine based single-use sensor for impedimetric detection of malathion. Anal Bioanal Chem 414, 1115–1128 (2022). https://doi.org/10.1007/s00216-021-03737-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03737-2