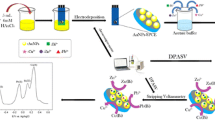
Abstract
The present work reports a newly developed square wave anodic strip** voltammetry (SWASV) methodology using novel gold nanostar–modified screen-printed carbon electrodes (AuNS/SPCE) and modified Britton-Robinson buffer (mBRB) for simultaneous detection of trace cadmium(II), arsenic(III), and selenium(IV). During individual and simultaneous detection, Cd2+, As3+, and Se4+ exhibited well-separated SWASV peaks at approximately − 0.48, − 0.09, and 0.65 V, respectively (versus Ag/AgCl reference electrode), which enabled a highly selective detection of the three analytes. Electrochemical impedance spectrum tests showed a significant decrease in charge transfer resistance with the AuNS/SPCE (0.8 kΩ) compared with bare SPCE (2.4 kΩ). Cyclic voltammetry experiments showed a significant increase in electroactive surface area with electrode modification. The low charge transfer resistance and high electroactive surface area contributed to the high sensitivity for Cd2+ (0.0767 μA (0.225 μg L−1)−1), As3+ (0.2213 μA (μg L−1)−1), and Se4+ (μA (μg L−1)−1). The three analytes had linear strip** responses over the concentration range of 0 to 100 μg L−1, with the obtained LoD for Cd2+, As3+, and Se4+ of 1.6, 0.8, and 1.6 μg L−1, respectively. In comparison with individual detection, the simultaneous detection of As3+ and Se4+ showed peak height reductions of 40.8% and 42.7%, respectively. This result was associated with the possible formation of electrochemically inactive arsenic triselenide (As2Se3) during the preconcentration step. Surface water analysis resulted in average percent recoveries of 109% for Cd2+, 93% for As3+, and 92% for Se4+, indicating the proposed method is accurate and reliable for the simultaneous detection of Cd2+, As3+, and Se4+ in real water samples.

Graphical abstract








Similar content being viewed by others
References
Mejáre M, Bülow L. Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol. 2001;19:67–73. https://doi.org/10.1016/s0167-7799(00)01534-1.
Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23:769–82. https://doi.org/10.1007/s10534-010-9343-z.
EPA, National Primary Drinking Water Regulations, 2009.
Majid E, Hrapovic S, Liu Y, Male KB, Luong JH. Electrochemical determination of arsenite using a gold nanoparticle modified glassy carbon electrode and flow analysis. Anal Chem. 2006;78:762–9. https://doi.org/10.1021/ac0513562.
Yokel RA, Lasley SM, Dorman DC. The speciation of metals in mammals influences their toxicokinetics and toxicodynamics and therefore human health risk assessment. J Toxicol Environ Health B Crit Rev. 2006;9:63–85. https://doi.org/10.1080/15287390500196230.
Luong JHT, Lam E, Male KB. Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal Methods. 2014;6:6157–69. https://doi.org/10.1039/c4ay00817k.
Segura R, Pizarro J, Díaz K, Placencio A, Godoy F, Pino E, et al. Development of electrochemical sensors for the determination of selenium using gold nanoparticles modified electrodes. Sensor Actuat B-Chem. 2015;220:263–9. https://doi.org/10.1016/j.snb.2015.05.016.
Piech R, Kubiak WW. Determination of trace selenium on hanging copper amalgam drop electrode. Electrochim Acta. 2007;53:584–9. https://doi.org/10.1016/j.electacta.2007.07.017.
Tan SH, Kounaves SP. Determination of selenium(IV) at a microfabricated gold ultramicroelectrode array using square wave anodic strip** voltammetry. Electroanalysis. 1998;10:364–8. https://doi.org/10.1002/(sici)1521-4109(199805)10:6<364::Aid-elan364>3.0.Co;2-f.
Sereshti H, Heravi YE, Samadi S. Optimized ultrasound-assisted emulsification microextraction for simultaneous trace multielement determination of heavy metals in real water samples by ICP-OES. Talanta. 2012;97:235–41. https://doi.org/10.1016/j.talanta.2012.04.024.
Huang C, Jiang Z, Hu B. Mesoporous titanium dioxide as a novel solid-phase extraction material for flow injection micro-column preconcentration on-line coupled with ICP-OES determination of trace metals in environmental samples. Talanta. 2007;73:274–81. https://doi.org/10.1016/j.talanta.2007.03.046.
Ranjbar L, Yamini Y, Saleh A, Seidi S, Faraji M. Ionic liquid based dispersive liquid-liquid microextraction combined with ICP-OES for the determination of trace quantities of cobalt, copper, manganese, nickel and zinc in environmental water samples. Microchim Acta. 2012;177:119–27. https://doi.org/10.1007/s00604-011-0757-2.
Narin I. Determination of trace metal ions by AAS in natural water samples after preconcentration of pyrocatechol violet complexes on an activated carbon column. Talanta. 2000;52:1041–6. https://doi.org/10.1016/S0039-9140(00)00468-9.
Rafiquel I, Jannat AF, Hasanuzzaman, Musrat R, Laisa AL, Dipak KP. Pollution assessment and heavy metal determination by AAS in waste water collected from Kushtia industrial zone in Bangladesh. Afr J Environ Sci Technol. 2016;10:9–17. https://doi.org/10.5897/AJEST2014.1994.
Zhao S, Liang H, Yan H, Yan Z, Chen S, Zhu X, et al. Online preconcentration and determination of trace levels cadmium in water samples using flow injection systems coupled with flame AAS. CLEAN - Soil, Air, Water. 2010;38:146–52. https://doi.org/10.1002/clen.200900228.
Li Y, Peng G, He Q, Zhu H, Al-Hamadani SM. Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim Acta A Mol Biomol Spectrosc. 2015;140:156–61. https://doi.org/10.1016/j.saa.2014.12.091.
Ammann AA. Speciation of heavy metals in environmental water by ion chromatography coupled to ICP-MS. Anal Bioanal Chem. 2002;372:448–52. https://doi.org/10.1007/s00216-001-1115-8.
El-Sewify IM, Shenashen MA, Shahat A, Yamaguchi H, Selim MM, Khalil MMH, et al. Ratiometric fluorescent chemosensor for Zn2+ ions in environmental samples using supermicroporous organic-inorganic structures as potential platforms. ChemistrySelect. 2017;2:11083–90. https://doi.org/10.1002/slct.201702283.
El-Sewify IM, Shenashen MA, Shahat A, Selim MM, Khalil MMH, El-Safty SA. Sensitive and selective fluorometric determination and monitoring of Zn2+ ions using supermicroporous Zr-MOFs chemosensors. Microchem J. 2018;139:24–33. https://doi.org/10.1016/j.microc.2018.02.002.
El-Sewify IM, Shenashen MA, Shahat A, Yamaguchi H, Selim MM, Khalil MMH, et al. Dual colorimetric and fluorometric monitoring of Bi3+ ions in water using supermicroporous Zr-MOFs chemosensors. J Lumin. 2018;198:438–48. https://doi.org/10.1016/j.jlumin.2018.02.028.
Toor SK, Devi P, Bansod BKS. Electrochemical detection of trace amount of arsenic (III) at glassy carbon electrode modified with au/Fe3O4 nanocomposites. Aqua Procedia. 2015;4:1107–13. https://doi.org/10.1016/j.aqpro.2015.02.140.
Chow E, Hibbert DB, Gooding JJ. Voltammetric detection of cadmium ions at glutathione-modified gold electrodes. Analyst. 2005;130:831–7. https://doi.org/10.1039/b416831c.
Li Y, Ma J, Ma Z. Synthesis of gold nanostars with tunable morphology and their electrochemical application for hydrogen peroxide sensing. Electrochim Acta. 2013;108:435–40. https://doi.org/10.1016/j.electacta.2013.06.141.
Dutta S, Strack G, Kurup P. Gold nanostar electrodes for heavy metal detection. Sensor Actuat B-Chem. 2019;281:383–91. https://doi.org/10.1016/j.snb.2018.10.111.
Ruecha N, Rodthongkum N, Cate DM, Volckens J, Chailapakul O, Henry CS. Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II). Anal Chim Acta. 2015;874:40–8. https://doi.org/10.1016/j.aca.2015.02.064.
Mafa PJ, Idris AO, Mabuba N, Arotiba OA. Electrochemical co-detection of As(III), Hg(II) and Pb(II) on a bismuth modified exfoliated graphite electrode. Talanta. 2016;153:99–106. https://doi.org/10.1016/j.talanta.2016.03.003.
Lu Y, Liang X, Xu J, Zhao Z, Tian G. Synthesis of CuZrO3 nanocomposites/graphene and their application in modified electrodes for the co-detection of trace Pb(II) and Cd(II). Sensor Actuat B-Chem. 2018;273:1146–55. https://doi.org/10.1016/j.snb.2018.06.104.
Bonfil Y, Kirowa-Eisner E. Determination of nanomolar concentrations of lead and cadmium by anodic-strip** voltammetry at the silver electrode. Anal Chim Acta. 2002;457:285–96. https://doi.org/10.1016/s0003-2670(02)00016-8.
Fischer E, van den Berg CMG. Anodic strip** voltammetry of lead and cadmium using a mercury film electrode and thiocyanate. Anal Chim Acta. 1999;385:273–80. https://doi.org/10.1016/s0003-2670(98)00582-0.
Armstrong KC, Tatum CE, Dansby-Sparks RN, Chambers JQ, Xue ZL. Individual and simultaneous determination of lead, cadmium, and zinc by anodic strip** voltammetry at a bismuth bulk electrode. Talanta. 2010;82:675–80. https://doi.org/10.1016/j.talanta.2010.05.031.
Kopanica M, Novotný L. Determination of traces of arsenic(III) by anodic strip** voltammetry in solutions, natural waters and biological material. Anal Chim Acta. 1998;368:211–8. https://doi.org/10.1016/s0003-2670(98)00220-7\.
** voltammetry. Anal Chim Acta. 2008;620:44–9. https://doi.org/10.1016/j.aca.2008.05.015.
Dai X, Nekrassova O, Hyde ME, Compton RG. Anodic strip** voltammetry of arsenic(III) using gold nanoparticle-modified electrodes. Anal Chem. 2004;76:5924–9. https://doi.org/10.1021/ac049232x.
Hamilton TW, Ellis J, Florence TM. Determination of selenium and tellurium in electrolytic copper by anodic strip** voltammetry at a gold film electrode. Anal Chim Acta. 1979;110:87–94. https://doi.org/10.1016/s0003-2670(01)83533-9.
Bryce DW, Izquierdo A, De Castro MDL. Flow-injection anodic strip** voltammetry at a gold electrode for selenium(IV) determination. Anal Chim Acta. 1995;308:96–101. https://doi.org/10.1016/0003-2670(94)00626-w.
Britton HTS, Robinson RA. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. Journal of the Chemical Society(Resumed). 1931:1456–62.
Yilmaz B, Kaban S. Electrochemical behavior of atorvastatin at glassy carbon electrode and its direct determination in pharmaceutical preparations by square wave and differential pulse voltammetry. Indian J Pharm Sci. 2016;78. https://doi.org/10.4172/pharmaceutical-sciences.1000126.
Süslü I, Altınöz SJ. Electrochemical behavior of quinapril and its determination in pharmaceutical formulations by square-wave voltammetry at a mercury electrode. Pharmazie. 2008;63:428–33. https://doi.org/10.1691/ph.2008.7387.
Ozkorucuklu SP, Sahin Y, Alsancak G. Voltammetric behaviour of sulfamethoxazole on electropolymerized-molecularly imprinted overoxidized polypyrrole. Sensors (Basel). 2008;8:8463–78. https://doi.org/10.3390/s8128463.
Yang C. Voltammetric determination of tinidazole using a glassy carbon electrode modified with single-wall carbon nanotubes. Anal Sci. 2004;20:821–4. https://doi.org/10.2116/analsci.20.821.
Chapin RE, Ku WW. The reproductive toxicity of boric acid. Environ Health Perspect. 1994;102(Suppl 7):87–91. https://doi.org/10.1289/ehp.94102s787.
Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E. 2010;42:1417–24. https://doi.org/10.1016/j.physe.2009.11.081.
Kalishwaralal K, Deepak V, Pandian SRK, Kottaisamy M, BarathmaniKanth S, Kartikeyan B, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B: Biointerfaces. 2010;77:257–62. https://doi.org/10.1016/j.colsurfb.2010.02.007.
Panicker CY, Varghese HT, Philip D, Nogueira HI. FT-IR, FT-Raman and SERS spectra of pyridine-3-sulfonic acid. Spectrochim Acta A Mol Biomol Spectrosc. 2006;64:744–7. https://doi.org/10.1016/j.saa.2005.06.048.
Philip D, Eapen A, Aruldhas G. Vibrational and surface enhanced Raman scattering spectra of sulfamic acid. J Solid State Chem. 1995;116:217–23. https://doi.org/10.1006/jssc.1995.1206.
Philip D. Honey mediated green synthesis of gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2009;73:650–3. https://doi.org/10.1016/j.saa.2009.03.007.
Smitha SL, Philip D, Gopchandran KG. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta A Mol Biomol Spectrosc. 2009;74:735–9. https://doi.org/10.1016/j.saa.2009.08.007.
Philip D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77:807–10. https://doi.org/10.1016/j.saa.2010.08.008.
Lether FG, Wenston PR. An algorithm for the numerical evaluation of the reversible Randles-Sevcik function. Comput Chem. 1987;11:179–83. https://doi.org/10.1016/0097-8485(87)80016-5.
Frankenthal RP. The anodic corrosion of gold in concentrated chloride solutions. J Electrochem Soc. 1982;129. https://doi.org/10.1149/1.2124085.
Cadle SH, Bruckenstein S. A ring-disk study of the effect of trace chloride ion on the anodic behavior of gold in 0.2 M H2SO4. J Electroanal Chem Interfacial Electrochem. 1973;48:325–31. https://doi.org/10.1016/s0022-0728(73)80365-1.
Xu X, Duan G, Li Y, Liu G, Wang J, Zhang H, et al. Fabrication of gold nanoparticles by laser ablation in liquid and their application for simultaneous electrochemical detection of Cd2+, Pb2+, Cu2+, Hg2+. ACS Appl Mater Interfaces. 2014;6:65–71. https://doi.org/10.1021/am404816e.
Jacobs ES. Anodic strip** voltammetry of gold and silver with carbon paste electrodes. Anal Chem. 1963;35:2112–5. https://doi.org/10.1021/ac60206a037.
Wei Y, Gao C, Meng F-L, Li H-H, Wang L, Liu J-H, et al. SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): an interesting favorable mutual interference. J Phys Chem C. 2011;116:1034–41. https://doi.org/10.1021/jp209805c.
Arsenic. Antimony and Bismuth. In: Greenwood NN, Earnshaw A, editors. Chemistry of the elements. Oxford: Butterworth-Heinemann; 1997. p. 547–99.
Welch AH, Helsel DR, Focazio MJ, Watkins SA. Arsenic in ground water supplies of the United States. In: Chappell WR, Abernathy CO, Calderon RL, editors. Arsenic exposure and health effects III. Oxford: Elsevier Science Ltd; 1999. p. 9–17.
Williamson JE, Carter JM. Water-Quality Characteristics in the Black Hills Area, South Dakota Water-Resources Investigations Report 01-4194. US Geological Survey, South Dakota Department of Environment and Natural Resources, West Dakota Water Development District. 2001.
Groschen GE, Arnold TL, Morrow WS, Warner KL. Occurrence and distribution of iron, manganese, and selected trace elements in ground water in the glacial aquifer system of the Northern United States. Geological Survey: U. S; 2009.
Anawar HM, Akai J, Komaki K, Terao H, Yoshioka T, Ishizuka T, et al. Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. J Geochem Explor. 2003;77:109–31. https://doi.org/10.1016/s0375-6742(02)00273-x.
EPA, SESD Operating Procedure, Surface water sampling, 2013.
Zhu L, Xu L, Huang B, Jia N, Tan L, Yao S. Simultaneous determination of Cd(II) and Pb(II) using square wave anodic strip** voltammetry at a gold nanoparticle-graphene-cysteine composite modified bismuth film electrode. Electrochim Acta. 2014;115:471–7. https://doi.org/10.1016/j.electacta.2013.10.209.
Funding
The authors received financial support from the U.S. Army Combat Capabilities Development Command – Soldier Center (Contract No. W911QY-17-2-0004) and the U.S. National Science Foundation Grant No. 1543042.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No informed consent, human participants, and animals applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Additional information
Distribution statement
This article has been approved for public release by the Public Affairs Office at the U.S. Army Combat Capabilities Development Command – Soldier Center (PAO No. U19-1565).
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, D., Sullivan, C., Brack, E.M. et al. Simultaneous voltammetric detection of cadmium(II), arsenic(III), and selenium(IV) using gold nanostar–modified screen-printed carbon electrodes and modified Britton-Robinson buffer. Anal Bioanal Chem 412, 4113–4125 (2020). https://doi.org/10.1007/s00216-020-02642-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02642-4