Abstract
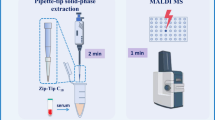
Quantification of ultra-trace analytes in complex biological samples using micro-solid-phase extraction followed by direct detection with internal extractive electrospray ionization mass spectrometry (μSPE–iEESI–MS) was demonstrated. 1-Hydroxypyrene (1-OHP) and papaverine at attomole levels in human raw urine samples were analyzed under negative and positive ion detection mode, respectively. The μSPE was simply prepared by packing a disposable syringe filter with octadecyl carbon chain (C18)-bonded micro silica particles, which were then treated as the “bulk sample” after the analytes were efficiently enriched by the C18 particles. Under the optimized experimental conditions, the analytes were readily eluted by isopropanol/water (80/20, V/V) at a high voltage of ± 4.0 kV, producing analyte ions under ambient conditions. The limit of detection (LOD) was 0.02 pg/L (9.2 amol) for 1-hydroxypyrene and 0.02 pg/L (5.9 amol) for papaverine. The acceptable linearity (R2 > 0.99), signal stability (RSD ≤ 10.7%), spike recoveries (91–95%), and comparable results for real urine samples were also achieved, opening up possibilities for quantitative analysis of trace compounds (at attomole levels) in complex bio-samples.

Graphical abstract




Similar content being viewed by others
References
Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;80(311):1566–70. https://doi.org/10.1126/science.1119426.
Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science (80-). 2004;306:471–3. https://doi.org/10.1126/science.1104404.
Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science (80- ). 2001;293:1289–92. https://doi.org/10.1126/science.1062711.
Deng J, Yang Y, Fang L, Lin L, Zhou H, Luan T. Coupling solid-phase microextraction with ambient mass spectrometry using surface coated wooden-tip probe for rapid analysis of ultra trace perfluorinated compounds in complex samples. Anal Chem. 2014;86:11159–66. https://doi.org/10.1021/ac5034177.
Reyes-Garcés N, Gionfriddo E, Gómez-Ríos GA, Alam MN, Boyacl E, Bojko B, et al. Advances in solid phase microextraction and perspective on future directions. Anal Chem. 2018;90:302–60. https://doi.org/10.1021/acs.analchem.7b04502.
Peng Z, Song W, Han F, Chen H, Zhu M, Chen Y. Chromatographic tandam mass spectrometric detection of papaverine and its major metabolites in rat urine. Int J Mass Spectrom. 2007;266:114–21. https://doi.org/10.1016/j.ijms.2007.07.013.
Dénes J, Katona M, Hosszú A, Czuczy N, Takáts Z. Analysis of biological fluids by direct combination of solid phase extraction and desorption electrospray ionization mass spectrometry. Anal Chem. 2009;81:1669–75. https://doi.org/10.1021/ac8024812.
Wang H, So PK, Yao ZP. Direct analysis of herbal powders by pipette-tip electrospray ionization mass spectrometry. Anal Chim Acta. 2014;809:109–16. https://doi.org/10.1016/j.aca.2013.11.060.
Deng J, Yang Y, Xu M, Wang X, Lin L, Yao ZP, et al. Surface-coated probe nanoelectrospray ionization mass spectrometry for analysis of target compounds in individual small organisms. Anal Chem. 2015;87:9923–30. https://doi.org/10.1021/acs.analchem.5b03110.
Ahmad S, Tucker M, Spooner N, Murnane D, Gerhard U. Direct ionization of solid-phase microextraction fibers for quantitative drug bioanalysis: from peripheral circulation to mass spectrometry detection. Anal Chem. 2015;87:754–9. https://doi.org/10.1021/ac503706n.
Ouyang G, Vuckovic D, Pawliszyn J. Nondestructive sampling of living systems using in vivo solid-phase microextraction. Chem Rev. 2011;111:2784–814. https://doi.org/10.1021/cr100203t.
Basheer C, Alnedhary AA, Rao BSM, Valliyaveettil S, Lee HK. Development and application of porous membrane-protected carbon nanotube micro-solid-phase extraction combined with gas chromatography/mass spectrometry. Anal Chem. 2006;78:2853–8. https://doi.org/10.1021/ac060240i.
López-Blanco MC, Cancho-Grande B, Simal-Gándara J. Comparison of solid-phase extraction and solid-phase microextraction for carbofuran in water analyzed by high- performance liquid chromatography–photodiode-array detection. J Chromatogr A. 2002;963:117–23.
Zhang C, Manicke NE. Development of a paper spray mass spectrometry cartridge with integrated solid phase extraction for bioanalysis. Anal Chem. 2015;87:6212–9. https://doi.org/10.1021/acs.analchem.5b00884.
Gómez-Ríos GA, Reyes-Garcés N, Bojko B, Pawliszyn J. Biocompatible solid-phase microextraction nanoelectrospray ionization: an unexploited tool in bioanalysis. Anal Chem. 2016;88:1259–65. https://doi.org/10.1021/acs.analchem.5b03668.
Hu B, So PK, Yao ZP. Electrospray ionization with aluminum foil: a versatile mass spectrometric technique. Anal Chim Acta. 2014;817:1–8. https://doi.org/10.1016/j.aca.2014.02.005.
Hu B, **n GZ, So PK, Yao ZP. Thin layer chromatography coupled with electrospray ionization mass spectrometry for direct analysis of raw samples. J Chromatogr A. 2015;1415:155–60. https://doi.org/10.1016/j.chroma.2015.08.055.
Spietelun A, Kloskowski A, Chrzanowski W, Namieśnik J. Understanding solid-phase microextraction: key factors influencing the extraction process and trends in improving the technique. Chem Rev. 2013;113:1667–85. https://doi.org/10.1021/cr300148j.
Yao ZP. Characterization of proteins by ambient mass spectrometry. Mass Spectrom Rev. 2012;31:437–47. https://doi.org/10.1002/mas.20346.
Chen H, Talaty NN, Takáts Z, Cooks RG. Desorption electrospray ionization mass spectrometry for high-throughput analysis of pharmaceutical samples in the ambient environment. Anal Chem. 2005;77:6915–27. https://doi.org/10.1021/ac050989d.
Wiseman JM, Ifa DR, Song Q, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew Chem Int Ed. 2006;45:7188–92. https://doi.org/10.1002/anie.200602449.
Venter A, Nefliu M, Graham Cooks R. Ambient desorption ionization mass spectrometry. TrAC Trends Anal Chem. 2008;27:284–90. https://doi.org/10.1016/j.trac.2008.01.010.
Chen H, Gamez G, Zenobi R. What can we learn from ambient ionization techniques? J Am Soc Mass Spectrom. 2009;20:1947–63. https://doi.org/10.1016/j.jasms.2009.07.025.
Cody RB, Laramée JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–302. https://doi.org/10.1021/ac050162j.
Chen H, Wortmann A, Zhang W, Zenobi R. Rapid in vivo fingerprinting of nonvolatile compounds in breath by extractive electrospray ionization quadrupole time-of-flight mass spectrometry. Angew Chem Int Ed. 2007;46:580–3. https://doi.org/10.1002/anie.200602942.
Hu L, Liang J, Chingin K, Hang Y, Wu X, Chen H. Early release of 1-pyrroline by pseudomonas aeruginosa cultures discovered using ambient corona discharge ionization mass spectrometry. RSC Adv. 2016;6:8449–55. https://doi.org/10.1039/c5ra24594j.
Chen H, Zheng J, Zhang X, Luo M, Wang Z, Qiao X. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J Mass Spectrom. 2007;42:1045–56. https://doi.org/10.1002/jms.1235.
Zhang X, Jia B, Huang K, Hu B, Chen R, Chen H. Tracing origins of complex pharmaceutical preparations using surface desorption atmospheric pressure chemical ionization mass spectrometry. Anal Chem. 2010;82:8060–70. https://doi.org/10.1021/ac100407k.
Chen H, Sun Y, Wortmann A, Gu H, Zenobi R. Differentiation of maturity and quality of fruit using noninvasive extractive electrospray ionization quadrupole time-of-flight mass spectrometry. Anal Chem. 2007;79:1447–55.
Chen H, Venter A, Cooks RG (2006) Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation. Chem Commun 2042–2044. https://doi.org/10.1039/b602614a.
Chen H, Yang S, Li M, Hu B, Li J, Wang J. Sensitive detection of native proteins using extractive electrospray ionization mass spectrometry. Angew Chem Int Ed. 2010;49:3053–6. https://doi.org/10.1002/anie.200906886.
Chen H, Yang S, Wortmann A, Zenobi R. Neutral desorption sampling of living objects for rapid analysis by extractive electrospray ionization mass spectrometry. Angew Chem Int Ed. 2007;46:7591–4. https://doi.org/10.1002/anie.200702200.
Chen H, Zenobi R. Neutral desorption sampling of biological surfaces for rapid chemical characterization by extractive electrospray ionization mass spectrometry. Nat Protoc. 2008;3:1467–75. https://doi.org/10.1038/nprot.2008.109.
Luo M, Hu B, Zhang X, Peng D, Chen H, Zhang L, et al. Spectrometry for sensitive detection of uranyl species in natural water samples. Anal Chem. 2010;82:282–9.
Zhang H, Lu H, Huang H, Liu J, Fang X, Yuan BF, et al. Quantification of 1-hydroxypyrene in undiluted human urine samples using magnetic solid-phase extraction coupled with internal extractive electrospray ionization mass spectrometry. Anal Chim Acta. 2016;926:72–8. https://doi.org/10.1016/j.aca.2016.04.033.
Nišavić M, Hozić A, Hameršak Z, Radić M, Butorac A, Duvnjak M, et al. High-efficiency microflow and nanoflow negative electrospray ionization of peptides induced by gas-phase proton transfer reactions. Anal Chem. 2017;89:4847–54. https://doi.org/10.1021/acs.analchem.6b04466.
Qi F, Qian L, Liu J, Li X, Lu L, Xu Q. A high-throughput nanofibers mat-based micro-solid phase extraction for the determination of cationic dyes in wastewater. J Chromatogr A. 2016;1460:24–32. https://doi.org/10.1016/j.chroma.2016.07.020.
So PK, Ng TT, Wang H, Hu B, Yao ZP. Rapid detection and quantitation of ketamine and norketamine in urine and oral fluid by wooden-tip electrospray ionization mass spectrometry. Analyst. 2013;138:2239–43. https://doi.org/10.1039/c3an36641c.
Yang S, Ding J, Zheng J, Hu B, Li J, Chen H, et al. Detection of melamine in milk products by surface desorption atmospheric pressure chemical ionization mass spectrometry. Anal Chem. 2009;81:2426–36. https://doi.org/10.1021/ac900063u.
Ramsauer B, Sterz K, Hagedorn H-W, Engl J, Scherer G, McEwan M, et al. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal Bioanal Chem. 2011;399:877–89. https://doi.org/10.1007/s00216-010-4355-7.
Lankova D, Urbancova K, Sram RJ, Hajslova J, Pulkrabova J. A novel strategy for the determination of polycyclic aromatic hydrocarbon monohydroxylated metabolites in urine using ultra-high-performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem. 2016;408:2515–25. https://doi.org/10.1007/s00216-016-9350-1.
Tang C, Tan J, Fan R, Zhao B, Tang C, Ou W, et al. Quasi-targeted analysis of hydroxylation-related metabolites of polycyclic aromatic hydrocarbons in human urine by liquid chromatography–mass spectrometry. J Chromatogr A. 2016;1461:59–69. https://doi.org/10.1016/j.chroma.2016.07.051.
Zhang H, Xu H. Electrospun nanofibers-based online micro-solid phase extraction for the determination of monohydroxy polycyclic aromatic hydrocarbons in human urine. J Chromatogr A. 2017;1521:27–35. https://doi.org/10.1016/j.chroma.2017.09.035.
Kang HG, Jeong SH. 1-OH-pyrene and 3-OH-phenanthrene in urine show good relationship with their parent polycyclic aromatic hydrocarbons in muscle in dairy cattle. Toxicol Res. 2011;27:15–8. https://doi.org/10.5487/TR.2011.27.1.015.
Li X, Zenobi R. Use of polyetheretherketone as a material for solid phase extraction of hydroxylated metabolites of polycyclic aromatic hydrocarbons in human urine. Anal Chem. 2013;85:3526–31. https://doi.org/10.1021/ac303402s.
Jacob P, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–98. https://doi.org/10.1021/ac060920l.
Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78:5744–51. https://doi.org/10.1021/ac0606094.
Musshoff F, Trafkowski J, Madea B. Validated assay for the determination of markers of illicit heroin in urine samples for the control of patients in a heroin prescription program. J Chromatogr B Anal Technol Biomed Life Sci. 2004;811:47–52. https://doi.org/10.1016/j.jchromb.2004.03.072.
Dams R, Benijts T, Lambert WE, De Leenheer AP. Simultaneous determination of in total 17 opium alkaloids and opioids in blood and urine by fast liquid chromatography-diode-array detection-fluorescence detection, after solid-phase extraction. J Chromatogr B Anal Technol Biomed Life Sci. 2002;773:53–61. https://doi.org/10.1016/S1570-0232(01)00594-3.
Kikura-Hanajiri R, Kaniwa N, Ishibashi M, Makino Y, Kojima S. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric analysis of opiates and metabolites in rat urine after inhalation of opium. J Chromatogr B Anal Technol Biomed Life Sci. 2003;789:139–50. https://doi.org/10.1016/S1570-0232(03)00096-5.
Lombardo-Agüí M, Cruces-Blanco C, García-Campaña AM. Capillary zone electrophoresis with diode-array detection for analysis of local anaesthetics and opium alkaloids in urine samples. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:833–6. https://doi.org/10.1016/j.jchromb.2009.01.041.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21605016, 21705017), International Science & Technology Cooperation Program of China (No. 2015DFA40290), and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (No. IRT_17R20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in this work involving human participants were in accordance with the ethical standards of the research committee of East China University of Technology and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants from the local Bureau of Drug Abuse Control (BDAC) included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 455 kb)
Rights and permissions
About this article
Cite this article
Han, J., Liu, W., Su, R. et al. Coupling of micro-solid-phase extraction and internal extractive electrospray ionization mass spectrometry for ultra-sensitive detection of 1-hydroxypyrene and papaverine in human urine samples. Anal Bioanal Chem 411, 3281–3290 (2019). https://doi.org/10.1007/s00216-019-01794-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01794-2