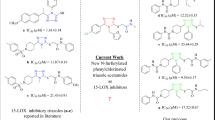
Abstract
The arachidonic acid metabolizing enzymes cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) have been considered to be associated with the occurrence and development of a variety of diseases, including inflammatory. Compared to classical NSAIDs, the dual COX-2/5-LOX inhibition is an effective strategy for designing compounds with more effective biological activities, fewer side effects, and a broader anti-inflammatory spectrum. In this study, we designed and synthesized a series of novel indole and indazole arylamide benzoic acid analogues as dual COX-2 / 5-LOX inhibitors and evaluated their anti-inflammatory properties in vivo. Compounds 7f and 7n showed significant anti-inflammatory activity in a xylene-induced mouse model of auricular edema. Furthermore, 7f and 7n exhibited moderate COX-2 inhibitory activity (IC50 = 537 and 321.5 nM) than celecoxib (IC50 = 10.04 nM) in vitro, among which 7n had higher COX-2 selectivity activity (selectivity index (COX-1/COX-2) = 7.89) and moderate 5-LOX inhibitory activity (IC50 = 222.1 nM). Compared to zileuton (IC50 = 36.46 nM), compound 7f was identified as the most potent 5-LOX inhibitor (IC50 = 77.37 nM). According to the biological results, compounds 7f and 7n have better inhibitory activities on the production of NO and PGE2 in LPS-induced RAW 264.7 cell macrophages than celecoxib and indomethacin. As demonstrated by docking studies, 7f and 7n have stronger interactions with key residues in the active pocket of COX-1 or COX-2, which is consistent with the activity results. Based on these results, further research into safer and more effective anti-inflammatory drugs might be possible using indole arylamide benzoic acid analogs.

Graphical abstract







Similar content being viewed by others
References
Liu J, Li DD, Dong W, Liu YQ, Wang YY, Chen YD. et al. Detection of an anti-angina therapeutic module in the effective population treated by a multi-target drug Danhong injection: a randomized trial. Signal Transduct Tar. 2021;6:329. https://doi.org/10.1038/s41392-021-00741-x.
Jan MS, Ahmad S, Hussain F, Ahmad A, Mahmood F, Rashid U. et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. Eur J Med Chem. 2020;186:111863. https://doi.org/10.1016/j.ejmech.2019.111863.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. https://doi.org/10.1038/nature07201.
Gouvea PD, Vasconcellos FA, Berwaldt GA, Fischer G, Sakata RP, Almeida WP. et al. 2-Aryl-3- (2-morpholinoethyl) thiazolidin-4-ones: Synthesis, anti-inflammatory in vivo, cytotoxicity in vitro and molecular docking studies. Eur J Med Chem. 2016;118:259–65. https://doi.org/10.1016/j.ejmech.2016.04.028.
Marsico F, Paolillo S, Filardi PP. NSAIDs and cardiovascular risk. J Cardiovasc Med. 2017;18:e40–e43. https://doi.org/10.2459/JCM.0000000000000443.
Tanaka KI, Suemasu S, Ishihara T, Tasaka Y, Arai Y, Mizushima T. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID) -dependent exacerbation of colitis. Eur J Med Chem. 2009;603:120–32. https://doi.org/10.1016/j.ejphar.2008.11.058.
Gaetano GD, Donati MB, Cerletti C. Prevention of thrombosis and vascular inflammation: benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol Sci. 2003;24:245–52. https://doi.org/10.1016/S0165-6147(03)00077-4.
Nguyen HT, Vu TY, Chandi V, Polimati H, Tatipamula UB. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep. 2020;10:15965. https://doi.org/10.1038/s41598-020-72840-8.
Sisa M, Dvorakova M, Temml V, Jarosova V, Vanek T, Landa P. Synthesis, inhibitory activity and in silico docking of dual COX/5-LOX inhibitors with quinone and resorcinol core. Eur J Med Chem. 2020;204:112620. https://doi.org/10.1016/j.ejmech.2020.112620.
Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–58.
Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. https://doi.org/10.1038/nrd1929.
Meshram MA, Bhise UO, Makhal PN, Kaki VR. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. Eur J Med Chem. 2021;225:113804. https://doi.org/10.1016/j.ejmech.2021.113804.
Singh P, Kaur J, Kaur H, Kaur A, Bhatti R. Synergy of physico-chemical and biological experiments for develo** a cyclooxygenase-2 inhibitor. Sci Rep. 2018;8:10005. https://doi.org/10.1038/s41598-018-28408-8.
Grosser T, Fries S, Fitzgerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. https://doi.org/10.1172/JCI27291.
Phoebe F, Waleed AM, Vaclav B, Novel. N-substituted indole Schiff bases as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase enzymes: Synthesis, biological activities in vitro and docking study. Eur J Med Chem. 2016;123:803–13. https://doi.org/10.1016/j.ejmech.2016.08.013.
Tran H, Márton, Rita M, Maul R, Baldermann S, Schreiner M. et al. Nasturtium (Indian cress, Tropaeolum majus nanum) dually blocks the COX and LOX pathway in primary human immune cells. Phytomedicine. 2016;23:611–20. https://doi.org/10.1016/j.phymed.2016.02.025.
Zoccal KF, Sorgi CA, Hori JI, Arantes EC, Serezani CH, Zamboni DS. et al. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat Commun. 2016;7:10760. https://doi.org/10.1038/ncomms10760.
Burnett BP, Levy RM. 5-lipoxygenase metabolic contributions to NSAID-Induced organ toxicity. Adv Ther. 2012;29:79–98. https://doi.org/10.1007/s12325-011-0100-7.
Sinha S, Manju SL, Doble M. Chalcone-thiazole hybrids: rational design, synthesis and lead identification against 5-lipoxygenase. Acs Med Chem Lett. 2019;10:1415–22. https://doi.org/10.1021/acsmedchemlett.9b00193.
Reginster J, Bias P, Buchner A. First clinical results of licofelone (ML3000), an inhibitor of COX-1, COX-2 and 5-LOX, for the treatment of osteoarthritis. Ann Rheum Dis. 2002;61:116.
Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–9. https://doi.org/10.1136/ard.62.6.501.
Zeeli S, Weill T, Finkin-Groner E, Bejar C, Melamed M, Furman S. et al. Synthesis and biological evaluation of derivatives of indoline as highly potent antioxidant and antiinflammatory agents. J Med Chem. 2018;61:4004–19. https://doi.org/10.1021/acs.jmedchem.8b00001.
Khanna S, Madan M, Vangoori A, Banerjee R, Thaimattam R, Ramesh M. et al. Evaluation of glycolamide esters of indomethacin as potential cyclooxygenase-2 (COX-2) inhibitors. Bioorgan Med Chem. 2006;14:4820–33. https://doi.org/10.1016/j.bmc.2006.03.023.
Neha K, Wakode S. Contemporary advances of cyclic molecules proposed for inflammation. Eur J Med Chem. 2021;221:113493 https://doi.org/10.1016/j.ejmech.2021.113493.
Kalgutkar AS, Crews BC, Saleh S, Prudhomme D, Marnett LJ. Indolyl esters and amides related to indomethacin are selective COX-2 inhibitors. Bioorgan Med Chem. 2006;13:6810–22. https://doi.org/10.1016/j.bmc.2005.07.073.
Singh P, Prasher P, Dhillon P, Bhatti R. Indole based peptidomimetics as anti-inflammatory and anti-hyperalgesic agents: Dual inhibition of 5-LOX and COX-2 enzymes. Eur J Med Chem. 2015;97:104–23. https://doi.org/10.1016/j.ejmech.2015.04.044.
Reddy MV, Billa VK, Pallela VR, Mallireddigari MR, Boominathan R, Gabriel JL. et al. Design, synthesis, and biological evaluation of 1- (4-sulfamylphenyl) -3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorgan Med Chem. 2008;16:3907–16. https://doi.org/10.1016/j.bmc.2008.01.047.
Huang YZ, Zhang B, Li JM, Liu HC, Zhang YC, Yang Z. et al. Design, synthesis, biological evaluation and docking study of novel indole-2-amide as anti-inflammatory agents with dual inhibition of COX and 5-LOX. Eur J Med Chem. 2019;180:41–50. https://doi.org/10.1016/j.ejmech.2019.07.004.
Eissa AM, Soliman EH, Khataibeh MH. Design, synthesis and anti-inflammatory activity of structurally simple anthranilic acid congeners devoid of ulcerogenic side effects. Chem Pharm Bull. 2012;60:1290–300. https://doi.org/10.1248/cpb.c12-00516.
Inglett GE, Chen D. Antioxidant and pasting properties of oat β-glucan hydrocolloids. Food Sci Nutr. 2012;3:827–35. https://doi.org/10.4236/fns.2012.36111.
Yi H, Hua J, Yun C, Wang XQ, Yang YQ, Tao JH. et al. Tranilast directly targets NLRP3 to treat inflammasome- driven diseases. Embo Mol Med. 2018;10:e8689. https://doi.org/10.15252/emmm.201708689.
Han SH, Suh HS, Jo H, Oh Y, Mishra NK, Han S. et al. Synthesis and anti-inflammatory evaluation of N-sulfonyl anthranilic acids via Ir(III) -catalyzed C-H amidation of benzoic acids. Bioorg Med Chem Lett. 2017;27:2129–34. https://doi.org/10.1016/j.bmcl.2017.03.072.
Narsinghani T, Sharma R. Synthesis, anti-inflammatory activities and docking studies of amide derivatives of meclofenamic acid. Chem Pap. 2016;71:857–68. https://doi.org/10.1007/s11696-016-0102-7.
Sharma S, Srivastava VK, Kumar A. Newer N-substituted anthranilic acid derivatives as potent anti-inflammatory agents - ScienceDirect. Eur J Med Chem. 2002;37:689–97. https://doi.org/10.1016/S0223-5234(02)01340-5.
Bruel A, Logé C, Marie-Ludivine DT, Ravache M, Guevel RL, Guillouzo C. et al. Synthesis and biological evaluation of new 5-benzylated 4-oxo-3,4-dihydro-5H-pyridazino [4,5-b] indoles as PI3Kα inhibitors. Eur J Med Chem. 2012;57:225–33. https://doi.org/10.1016/j.ejmech.2012.09.001.
Yuan Y, Rosado-Lugo JD, Zhang YZ, Datta P, Sun YS, Cao YL. et al. Evaluation of heterocyclic carboxamides as potential efflux pump inhibitors in pseudomonas aeruginosa. Antibiotics. 2022;11:30–46. https://doi.org/10.3390/antibiotics11010030.
Romagnoli R, Prencipe F, Oliva P, Salvador MK, Brancale A, Ferla S. et al. Design, synthesis and biological evaluation of 2-Alkoxycarbonyl-3-Anilinoindoles as a new class of potent inhibitors of tubulin polymerization. Bioorg Chem. 2020;97:103665. https://doi.org/10.1016/j.bioorg.2020.103665.
Parker AN, Martin MC, Shenje R, France S. Calcium-catalyzed formal [5+2] cycloadditions of alkylidene β-ketoesters with olefins: chemodivergent synthesis of highly functionalized cyclohepta [b] indole derivatives. Org Lett. 2019;21:7268–73. https://doi.org/10.1021/acs.orglett.9b02498.
Szabó T, Papp M, Németh DR, Dancso A, Volk B, Milen M. Synthesis of Indolo [2,3-c] quinolin-6 (7H) -ones and Antimalarial Isoneocryptolepine. Computational Study on the Pd-Catalyzed Intramolecular C-H Arylation. J Org Chem. 2021;86:128–45. https://doi.org/10.1021/acs.joc0c01832.
Shi Y, Duan YH, Ji YY, Wang ZL, Wu YR, Gunosewoyo H. et al. Amidoalkylindoles as Potent and Selective Cannabinoid Type 2 Receptor Agonists with in vivo efficacy in a mouse model of multiple sclerosis. J Med Chem. 2017;60:7067–83. https://doi.org/10.1021/acs.jmedchem.7b00724.
Kulmacz RJ, Lands W. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins. 1983;25:531–40. https://doi.org/10.1016/0090-6980(83)90025-4.
Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–8. https://doi.org/10.1038/384644a0.
Morris GM, Huey R, Olson AJ. Using AutoDock for ligand‐receptor docking. Curr Protoc Bioinforma. 2008;8:8–14. https://doi.org/10.1002/0471250953.bi0814s24.
Lill MA, Danielson ML. Computer-aided drug design platform using PyMOL. J Comput Aid Mol Des. 2010;25:13–9. https://doi.org/10.1007/s10822-010-9395-8.
Derek AW, Adrian RM, Paul RC. COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet . 2000;355:646–8. https://doi.org/10.1016/s0140-6736(99)12031-2.
He ZL, Tao D, **ong JM, Lou FF, Zhang JY, Chen JX. et al. Phosphorylation of 5-LOX: The Potential Set-point of Inflammation. Neurochem Res. 2020;45:2245–57. https://doi.org/10.1007/s11064-020-03090-3.
Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. https://doi.org/10.1161/ATVBAHA.110.207449.
Jaismy JP, Manju SL, Ethiraj KR, Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur J Pharm Sci. 2018;S0928-0987:30259–8. https://doi.org/10.1016/j.ejps.2018.06.003.
Abdelazeem AH, Gouda AM, Omar HA, Tolba MF. Design, synthesis and biological evaluation of novel diphenylthiazole-based cyclooxygenase inhibitors as potential anticancer agents. Bioorg Chem. 2014;57:132–41. https://doi.org/10.1016/j.bioorg.2014.10.001.
Cingolani G, Panella A, Perrone MG, Vitale P, Mauro GD, Fortuna CG. et al. Structural basis for selective inhibition of Cyclooxygenase-1 (COX-1) by diarylisoxazoles mofezolac and 3- (5-chlorofuran-2-yl) -5-methyl-4-phenylisoxazole (P6). Eur J Med Chem. 2017;138:661–8. https://doi.org/10.1016/j.ejmech.2017.06.045.
Mohamed MS, Mansour YE, Amin HK, EI-Araby ME. Molecular modelling insights into a physiologically favourable approach to eicosanoid biosynthesis inhibition through novel thieno [2,3-b] pyridine derivatives. J Enzym Inhib Med Ch. 2018;33:755–67. https://doi.org/10.1080/14756366.2018.1457657.
Tzvetkov NT, Stammler HG, Hristova S, Atanasov AG, Antonov L. (Pyrrolo-pyridin-5-yl) benzamides: BBB permeable monoamine oxidase B inhibitors with neuroprotective effect on cortical neurons. Eur J Med Chem. 2019;162:793–809. https://doi.org/10.1016/j.ejmech.2018.11.009.
Lei PA, Cyl B, Ghg B, Quan ZS. Synthesis, in vitro and in vivo biological evaluation of novel lappaconitine derivatives as potential anti-inflammatory agents-ScienceDirect. Acta Pharm Sin B. 2020;10:628–45. https://doi.org/10.1016/j.apsb.2019.09.002.
Labib MB, Fayez AM, El-Nahass ES, Awadallah M, Halim PA. Novel tetrazole-based selective COX-2 inhibitors: Design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg Chem. 2020;104:104308. https://doi.org/10.1016/j.bioorg.2020.104308.
Yon R, Long WY, Lai ZH, Sha L, Wu K, Yu X. et al. Discovery of a potential anti-inflammatory agent: 3-Oxo-29-noroleana-1,9 (11), 12-trien-2, 20-dicarbonitrile. J Med Chem. 2013;56:1984–95. https://doi.org/10.1021/jm301652t.
Funding
An exploratory scientific research project of Anhui University of Traditional Chinese Medicine(No:2021hxts06), and project supported by the Natural Science Foundation of Anhui Province(1508085MB3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, L., Du, S., Li, J. et al. Design, synthesis, and biological evaluation of dual-target COX-2/5-LOX inhibitors for the treatment of inflammation. Med Chem Res 32, 218–238 (2023). https://doi.org/10.1007/s00044-022-02995-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02995-8