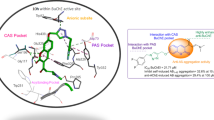
Abstract
Polyfunctional compounds comprise a novel class of therapeutic agents for the treatment of multi-factorial diseases. A series of 2-Phenyl-4H-chromen-4-one and its derivatives (5a–n) were designed, synthesized, and evaluated for their poly-functionality against acetylcholinestrase (AChE) and advanced glycation end products (AGEs) formation inhibitors against Alzheimer’s disease (AD). The screening results showed that most of them exhibited a significant ability to inhibit AChE AGEs formation with additional radical scavenging activity. Especially, 5m, 5b, and 5j displayed the greatest ability to inhibit AChE (IC50 = 8.0, 8.2, and 11.8 nM, respectively) and AGEs formation (IC50 = 55, 79, and 54 µM, respectively) with good antioxidant activity. Molecular docking studies explored the detailed interaction pattern with active, peripheral, and mid-gorge sites of AChE. These compounds, exhibiting such multiple pharmacological activities, can be further taken a lead for the development of potent drugs for the treatment of Alzheimer’s disease.






Similar content being viewed by others
Abbreviations
- ChE:
-
Cholinesterase
- AD:
-
Alzheimer’s disease
- AChE:
-
Acetylcholinesterase
- AGEs:
-
Advanced glycation end products
- CAS:
-
Catalytic active site
- PAS:
-
Peripheral anionic site
- ACh:
-
Acetylcholine
- FDA:
-
Food and drug administration
- OS:
-
Oxidative stress
- ROS:
-
Reactive oxygen species
- Aβ:
-
β-amyloid
- RAGE:
-
Receptor for AGEs
- DPPH:
-
1,1-diphenyl-2-picryl-hydrazyl
- EWG:
-
Electron withdrawing groups
References
Lee J, Yu J, Son SH, Heo J, Kim T, An JY, Inn KS, Kim NJ (2016) A versatile approach to flavones via a one-pot Pd(II)-catalyzed dehydrogenation/oxidative boron-Heck coupling sequence of chromanones. Org Biomol Chem 14:777–784
Cabrera M, Simoens M, Falchi G, Lavaggi ML, Piro OE, Castellano EE, Vidal A, Azqueta A, Monge A, de Ceráin AL, Sagrera G, Seoane G, Cerecetto H, González M (2007) Synthetic chalcones, flavanones, and flavones as antitumoral agents: biological evaluation and structure–activity relationships. Bioorg Med Chem 15:3356–3367
Cárdenas M, Marder M, Blank VC, Roguin LP (2006) Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem 14:2966–2971
Narwal M, Haikarainen T, Fallarero A, Vuorela PM, Lehtio L (2013) Screening and structural analysis of flavones inhibiting tankyrases. J Med Chem 56:3507–3517
Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, Chen FC (2002) Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem 10:2795–2802
Dao TT, Chi YS, Kim J, Kim HP, Kim S, Park H (2004) Synthesis and inhibitory activity against COX-2 catalyzed prostaglandin production of chrysin derivatives. Bioorg Med Chem Lett 14:1165–1167
Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW (1994) Inhibition of HIV-1 Integrase by flavones, caffeic acid phenethyl ester (Cape) and related compounds. Biochem Pharmacol 48:595–608
Baker W (1933) Molecular rearrangement of some o-acyloxyacetophenones and the mechanism of the production of 3-acylchromones. J Chem Soc 10:1381–1389
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Bolognesi ML, Rosini M, Andrisano V, Bartolini M, Minarini A, Tumiatti V, Melchiorre C (2009) MTDL design strategy in the context of Alzheimer’s disease: from lipocrine to memoquin and beyond. Curr Pharm Des 15:601–613
Ellman GL, Courtney KD, Valentino A, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fang L, Kraus B, Lehmann J, Heilmann J, Zhang Y, Decker M (2008) Design and synthesis of tacrine-ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg Med Chem Lett 18:2905–2909
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Glide (2010) Version 5.6. Schrodinger LLC, New York
Jung M, Park M (2007) Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 12:2130–2139
Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, Lee SE (2005) Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem 53:8537–8541
Kryger G, Silman I, Sussman JL (1999) Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure 7:297–307
Ligprep (2011) Version 2.5. Schrodinger LLC, New York
Lim SS, Han SM, Kim SY, Bae YS, Kang IJ (2007) Isolation of acetylcholinesterase inhibitors from the flowers of chrysanthemum indicum linne. Food Sci Biotech 16:265–269
Mahal HS, Venkataraman KJ (1934) Synthetical experiments in the chromone group. Part XIV. The action of sodamide on 1-acyloxy-2-acetonaphthones. J Chem Soc 56:1767–1769
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147
Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J, Ubukata M (2002) Screening system for the maillard reaction inhibitor from natural product extracts. J Health Sci 48:520–526
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127
Morphy R, Rankovic Z (2005) Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem 48:6523–6543
Münch Gl, Thome J, Foley P, Schinzel R, Riederer P (1997) Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res Rev 23:134–143
Pi RB, Ye MZ, Cheng ZY, Liu PQ (2008) Univ Zhongshan (UZHO-C). Patent CN101284812-A, China
Prathapan A, Nampoothiri SV, Mini S, Raghu KG (2012) Antioxidant, antiglycation and inhibitory potential of Saraca ashoka flowers against the enzymes linked to type 2 diabetes and LDL oxidation. Eur Rev Med Pharmacol Sci 16:57–65
Schmitt B, Bernhardt T, Moeller HJ, Heuser I, Frolich L (2004) Combination therapy in Alzheimer’s disease: a review of current evidence. CNS Drugs 18:827–844
Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, Singh D (2013) Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem 70:165–188
Singh M, Kaur M, Silakari O (2014) Flavones: an important scaffold for medicinal chemistry. Eur J Med Chem 84:206–239
Singh M, Kaur M, Chadha N, Silakari O (2016) Hybrids: a new paradigm to treat Alzheimer’s disease. Mol Divers 20:271–297
Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA (1994) Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA 91:7787–7791
Yamagishi S, Takeuchi M, Inagaki Y, Nakamura K, Imaizumi T (2003) Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmacol Res 23:129–134
Youdim MB, Buccafusco JJ (2005) Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci 26:27–35
Zhu JT, Choi RC, Chu GK, Cheung AW, Gao QT, Li J, Jiang ZY, Dong TT, Tsim KW (2007) Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death. J Agric Food Chem 55:2438–2445
Acknowledgements
We acknowledge the financial support from the “Indian Council of Medical Research (ICMR)”, New Delhi, for providing us Senior Research Fellowships (ICMR-SRF); Award nos. BIC/11(11)/2014 and BIC/11(02)/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Singh, M., Kaur, M., Vyas, B. et al. Design, synthesis and biological evaluation of 2-Phenyl-4H-chromen-4-one derivatives as polyfunctional compounds against Alzheimer’s disease. Med Chem Res 27, 520–530 (2018). https://doi.org/10.1007/s00044-017-2078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-2078-4