Abstract
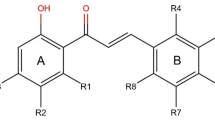
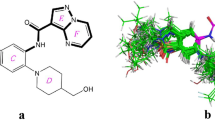
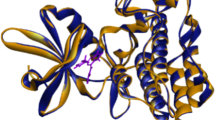
In this paper, a three-dimensional quantitative structure–activity relationships study for 38 HIV-1 protease inhibitors was established using Topomer CoMFA. The multiple correlation coefficients of fitting, cross-validation, and external validation were 0.959, 0.867, and 0.964, respectively. The results indicated that the model obtained has both favorable estimation stability and good prediction capability. Topomer Search was used to search R-group from ZINC database. As the result, a series of R-groups with relatively high activity contribution was obtained. By No. 32 molecule filtering, 10 Ra and 6 Rb groups were selected. We employed the 10 Ra and 6 Rb groups to alternately substitutes for the Ra and Rb of sample 32. Finally, we designed 60 new compounds with higher activity than that of the template molecule. The results suggested the Topomer Search technology could be effectively used to screen and design new HIV-1 protease inhibitors and has good predictive capability to guide the design of new HIV/AIDS drugs. As the ligands, the training molecules and new designed compounds were used for molecular docking to study the interaction mode with the protein receptor. As the results, we found that the ligands would form the hydrogen-bonding interactions with Asp25, Asp29, Asp30, Gly48, and Ile50 of the protein receptor generally.







Similar content being viewed by others
References
Ali A, Kiran Kumar Reddy GS, Cao H, Anjum SG, Nalam MNL, Schiffer CA, Rana TM (2006) Discovery of HIV-1 protease inhibitors with picomolar affinities incorporating N-aryl-oxazolidinone-5-carboxamides as novel P2 ligands. J Med Chem 49:7342–7356
Bhole RP, Bhusari KP (2011) Synthesis antihypertensive activity, and 3D-QSAR studies of some new p-hydroxybenzohydrazide derivatives. Archiv Der Pharmazie 344:119–134
Caldarini M, Sonar P, Valpapuram I, Tavella D, Volonté C, Pandini V, Vanoni MA, Aliverti A, Broglia RA, Tiana G, Cecconi C (2014) The complex folding behavior of HIV-1-protease monomer revealed by optical-tweezer single-molecule experiments and molecular dynamics simulations. Biophys Chem 195:32–42
Cramer RD (2003) Topomer CoMFA: a design methodology for rapid lead optimization. J Med Chem 46:374–388
Cramer RD, Cruz P, Stahl G, Curtiss WC, Campbell B, Masek BB, Soltanshahi F (2008) Virtual screening for R-groups, including predicted pIC50 contributions, within large structural databases, using Topomer CoMFA. J Chem Inf Model 48:2180–2195
Ghosh AK, Kincaid JF, Cho W, Walters DE, Krishnan K, Hussain KA, Koo Y, Cho H, Rudall C, Holland L, Buthod J (1998) Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino) sulfonamide isostere. Bioorg Med Chem Lett 8:687–690
Global Report (2012) UNAIDS report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva
Jadhav PK, Ala P, Woerner FJ, Chang CH, Garber SS, Anton ED, Bacheler LT (1997) Cyclic urea amides: HIV-1 protease inhibitors with low nanomolar potency against both wild type and protease inhibitor resistant mutants of HIV. J Med Chem 40:181–191
Ji YJ, Shu M, Lin Y, Wang YQ, Wang R, Hu Y, Lin ZH (2013) Combined 3D-QSAR modeling and molecular docking study on azacycles CCR5 antagonists. J Mol Struct 1045:35–41
Kiso Y, Matsumoto H, Yamaguchi S, Kimura T (1999) Design of small peptidomimetic HIV-1 protease inhibitors and prodrug forms. Lett Pept Sci 6:275–281
Liu HH, Golin CE, Miller LG, Hays RD, Keith Beck C, Sanandaji S, Christian J, Maldonado T, Duran D, Kaplan AH, Wenger NS (2001) A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 134:968–977
Miao X (2013) Molecular design targeting on Tau protein for Alzheimer’s disease. Chongqing University, Chongqing
Moore JP, Kitchen SG, Pugach P, Zack JA (2004) The CCR5 and CXCR4 coreceptors-central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retrov 20:111–126
Reeves JD, Piefer AJ (2005) Emerging drug targets for antiretroviral therapy. Drugs 65:1747–1766
Roy K, Leonard JT (2004) QSAR modeling of HIV-1 reverse transcriptase inhibitor 2-amino-6-arylsulfonylbenzonitriles and congeners using molecular connectivity and E-state parameters. Bioorgan Med Chem 12:745–754
Sharma MC (2014) Structural requirements of N-aryl-oxazolidinone-5-carboxamidederivatives for anti-HIV protease activity using molecular modelling techniques. J Taibah Univ Sci 8:111–123
Surleraux DLNG, Tahri A, Verschueren WG, Pille GME, de Kock HA, Jonckers THM, Peeters A, De Meyer S, Azijn H, Pauwels R, de Bethune MP, King NM, Prabu-Jeyabalan M, Schiffer CA, Wigerinck PBTP (2005) Discovery and selection of TMC114, a next generation HIV-1 protease inhibitor. J Med Chem 48:1813–1822
Thaisrivongs S, Strohbach JW (1999) Structure-based discovery of tipranavir disodium (PNU-140690E): a potent, orally bioavailable, nonpeptidic HIV ptotease inhibitor. Biopolymers 51:51–58
Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA (2012) Antiretroviral treatment of adult HIV infection: 2012 recommendations of the international antiviral society-USA panel. J Am Med Assoc 308:387–402
Tsantrizos YS (2008) Peptidomimetic therapeutic agents targeting the protease enzyme of the human immunodeficiency virus and hepatitis C virus. Acc Chem Res 41:1252–1263
Xu L, Shao XG (2004) Methods of chemometrics. Science Press, Bei**g, 166–169
Acknowledgments
This work was supported by the National Natural Science Funds of China (21475081)(21275094), the Natural Science Foundation of Shaanxi Province of China (2015JM2057), and the Graduate Innovation Fund of Shaanxi University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tong, JB., Bai, M. & Zhao, X. 3D-QSAR and docking studies of HIV-1 protease inhibitors using R-group search and Surflex-dock. Med Chem Res 25, 2619–2630 (2016). https://doi.org/10.1007/s00044-016-1701-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1701-0