Abstract
The endogenous mitochondrial quality control (MQC) system serves to protect mitochondria against cellular stressors. Although mitochondrial dysfunction contributes to cardiac damage during many pathological conditions, the regulatory signals influencing MQC disruption during septic cardiomyopathy (SC) remain unclear. This study aimed to investigate the involvement of pyruvate kinase M2 (PKM2) and prohibitin 2 (PHB2) interaction followed by MQC impairment in the pathogenesis of SC. We utilized LPS-induced SC models in PKM2 transgenic (PKM2TG) mice, PHB2S91D-knockin mice, and PKM2-overexpressing HL-1 cardiomyocytes. After LPS-induced SC, cardiac PKM2 expression was significantly downregulated in wild-type mice, whereas PKM2 overexpression in vivo sustained heart function, suppressed myocardial inflammation, and attenuated cardiomyocyte death. PKM2 overexpression relieved sepsis-related mitochondrial damage via MQC normalization, evidenced by balanced mitochondrial fission/fusion, activated mitophagy, restored mitochondrial biogenesis, and inhibited mitochondrial unfolded protein response. Docking simulations, co-IP, and domain deletion mutant protein transfection experiments showed that PKM2 phosphorylates PHB2 at Ser91, preventing LPS-mediated PHB2 degradation. Additionally, the A domain of PKM2 and the PHB domain of PHB2 are required for PKM2-PHB2 binding and PHB2 phosphorylation. After LPS exposure, expression of a phosphorylation-defective PHB2S91A mutant negated the protective effects of PKM2 overexpression. Moreover, knockin mice expressing a phosphorylation-mimetic PHB2S91D mutant showed improved heart function, reduced inflammation, and preserved mitochondrial function following sepsis induction. Abundant PKM2 expression is a prerequisite to sustain PKM2-PHB2 interaction which is a key element for preservation of PHB2 phosphorylation and MQC, presenting novel interventive targets for the treatment of septic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a severe complication of sepsis, septic cardiomyopathy (SC) manifests as reversible myocardial depression in patients with septic shock. Clinical features of SC include left ventricular dilation, decreased myocardial contractile function, and increased end-diastolic ventricular volume. Inflammatory injury, aberrant immune response, and cytokine storm have been proposed as the molecular bases of decreased myocardial function during sepsis-induced cardiomyopathy [1, 2]. However, findings from a randomized study in patients with virus-negative inflammatory cardiomyopathy suggest that immunosuppressive strategies may not provide additional clinical benefits for patients with SC [3, Full size image
In line with these findings, LPS-mediated PHB2 downregulation was nullified upon incubation of HL-1 cardiomyocytes with the proteasome inhibitor MG132 (Fig. 6F-G), suggesting involvement of the ubiquitin-proteasome system. On the contrary, and confirming proteasome involvement, PKM2 overexpression failed to sustain PHB2 expression in LPS-treated cardiomyocytes pre-incubated with betulinic acid (BA), a potent proteasome activator (Fig. 6H-I). Accordingly, protein stability analysis using pulse-chase assays showed that MG132 exposure counteracted LPS-stimulated PHB2 degradation (Fig. 6J), whereas PKM2 overexpression failed to extend the half-life of PHB2 in BA-treated cardiomyocytes (Fig. 6K). These results suggested that PKM2 overexpression in cardiomyocytes attenuates proteasome-mediated PHB2 degradation.
To elucidate the molecular basis underlying PKM2-mediated PHB2 stability, we evaluated the presence of constitutive interactions between PKM2 and PHB2 in cultured HL-1 cells. First, the active regions of PKM2 and PHB2 were screened by molecular docking simulations. We detected the existence of H-bond or hydrophobic interactions between PKM2 and PHB2 (Fig. 6L and M), with a minimum binding energy of -12.3 kcal·mol− 1. Co-IP assays further confirmed an endogenous interaction between PKM2 and PHB2 under physiological condition or LPS stress (Fig. 6N and O). To define the structural moieties required for PKM2/PHB2 binding, we first mapped the regions of PKM2 that are required for protein-protein binding. Since PKM2 has four domains, we generated four different domain-deletion PKM2 mutants (Fig. 6P) and transfected them into HL-1 cardiomyocytes. Co-IP assays showed that PKM2ΔA, PKM2ΔC, and PKM2ΔN, but not PKM2ΔB, were able to pull-down PHB2 (Fig. 6Q).
PKM2 induces PHB2 phosphorylation
After we revealed an interaction between PKM2 and PHB2, we asked whether conformational changes occur in PHB2 for its stabilization after PKM2 binding. To address this question, we analyzed the regions of PHB2 that are required for its interaction with PKM2. As PHB2 has four major domains, we generated four different domain deletion PHB2 mutants (Fig. 7A) and transfected them into HL-1 cardiomyocytes. Co-IP assays showed that PHB2ΔN, PHB2ΔCC, and PHB2ΔC, but not PHB2ΔPHB, were able to pull-down PKM2 (Fig. 7B). This suggests that the PHB domain of PHB2 determines its binding to PKM2.
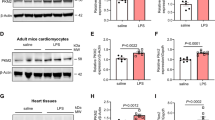
PKM2 induces PHB2 phosphorylation. PKM2 transgenic (PKM2Tg) mice and wild-type (WT) mice aged 8–10 weeks were injected intraperitoneally with 10 mg/kg LPS to induce SC. In vivo measurements were performed after 24 h later, and mice administered an equal volume of phosphate buffer saline served as controls. Immortalized mouse cardiac muscle HL-1 cells were treated with 10 µg/mL of LPS for 24 h to simulate SC in vitro. Cells treated with an equal volume of phosphate buffer saline were used as controls. Before LPS treatment, cardiomyocytes were transduced with Adenovirus encoding PKM2 (Ad-PKM2). β-gal-overexpressing (Ad-β-gal) cells were used as controls. (A) Map** of PHB2 regions and deletion mutants. (B) Representative immunoblot from immunoprecipitation analysis of HL-1 cells transfected with PHB2 deletion mutants. (C-F) Co-IP analysis of the interaction between dimeric and tetrameric PKM2 forms and PHB2 in HL-1 cells. (G-I) Western blot analysis of p-PHB2S176 and p-PHB2Ser91 expression in cardiac tissues. (J, K) Representative results of an in vitro kinase assay evaluating binding of phospho-PHB2 variants to PKM2 in the presence of LPS or compound 3k, a PKM2 inhibitor. (L, M) Pulse-chase analysis of PHB2 protein half-life in HL-1 cells transfected with PHB2S91D and PHB2S91A mutant constructs. Values are presented as mean ± SEM. For in vivo data, n = 6 mice per group. For in vitro data, n = 4 independent experiments. #p < 0.05, and ##p < 0.01
PKM2 has two conformations, a tetrameric one with high protein kinase (PK) activity that catalyzes the production of pyruvate from phosphoenolpyruvate (PEP), and a dimeric one, which has lower PK activity and also exerts non-glycolytic enzymatic functions. Interestingly, co-IP results showed that PHB2 mainly interacted with dimeric, rather than tetrameric, PKM2 (Fig. 7C and F). Thus, LPS seems to interrupt the cooperation between PHB2 and dimeric PKM2. These results hinted that the kinase function of PKM2 is involved in the regulation of the PHB domain of PHB2. PKM2 has been reported to directly induce post-transcriptional phosphorylation of several proteins, such as ERK2T202 [37], β-cateninY333 [38], histone H3T11 [39], myosin light chain 2Y118 [40], and protein kinase B substrate l (AKT1S1)S202 [41]. On the other hand, PHB2 possesses three phosphorylation sites (S91, S176, and S243) which have been reported to enhance the anti-oxidative and anti-apoptotic properties of PHB2 through an undefined mechanism [42,43,44]. Since the PHB domain of PHB2 encompasses amino acids 68–185, we asked whether PKM2/PHB2 interaction results in PHB2 phosphorylation within this sequence fragment. Western blotting showed abundant expression of p-PHB2S91 and little p-PHB2S176 in heart tissues under baseline conditions. Neither LPS nor PKM2 overexpression had an impact on p-PHB2S176 expression (Fig. 7G and I). In contrast, after exposure to LPS the expression of p-PHB2S91 was downregulated in WT mice but remained at near normal levels in PKM2Tg mice, compared with PBS treated mice (Fig. 7G and I). An in vitro kinase assay further showed that PKM2 induced PHB2 phosphorylation at S91, and this alteration was significantly inhibited by compound 3k, a PKM2 inhibitor (Fig. 7J and K).
To evaluate whether PKM2-mediated PHB2 phosphorylation at S91 is necessary for PHB2 stabilization, phosphorylation-defective (PHB2S91A) and phosphorylation-mimetic (PHB2S91D) PHB2 mutants were transfected into HL-1 cells. After transfection with PHB2S91D, LPS-mediated PHB2 degradation was delayed in control cells (Fig. 7L and M). In turn, in cells transfected with a PHB2S91A mutant, PKM2 overexpression failed to maintain PHB2 stability (Fig. 7L and M). Taken together, our results showed that PKM2 binds to PHB2 and phosphorylates it at S91, leading to increased PHB2 stability.
PHB2 dephosphorylation abolishes PKM2-mediated mitochondrial protection
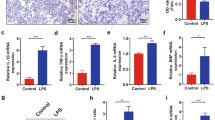
To evaluate whether PHB2 phosphorylation underlies PKM2-related MQC activation during SC, further assays were conducted in HL-1 cells transfected with PHB2S91A and PHB2S91D mutants. Immunofluorescent assessment of mitochondrial morphology showed that LPS-induced mitochondrial fragmentation was attenuated by overexpression of either PHB2S91D or PKM2 (Fig. 8A and C). However, in HL-1 cells transfected with PHB2S91A, PKM2 overexpression failed to maintain mitochondrial length and reduce the number of cardiomyocytes with plenty of fragmented mitochondria (Fig. 8A and C). Further, western blot analysis showed that LPS-mediated disruption of mitochondrial fission/fusion and mitophagy could be normalized by overexpression of PHB2S91D or PKM2 (Fig. 8D and J). In contrast, in HL-1 cells transfected with PHB2S91A, PKM2 overexpression failed to sustain mitochondrial fission/fusion and mitophagy (Fig. 8D and J). Similarly, mt-Keima assays confirmed that either PHB2S91D or PKM2 overexpression was able to sustain mitophagy in the presence of LPS, while PKM2-induced mitophagy was nullified by PHB2S91A transfection (Fig. 8K and L). Furthermore, mitochondrial biogenesis was stimulated by PHB2S91D or PKM2 overexpression in LPS-treated cells, while the effect of PKM2 overexpression was abrogated by PHB2S91A transfection (Fig. 8M and N). Lastly, transcriptions of mtUPR-related genes were slightly increased by LPS and partially restored toward baseline levels upon overexpression of either PHB2S91D or PKM2 (Fig. 8O-P). However, in cells transfected with the PHB2S91A mutant, the inhibitory effect of PKM2 on mtUPR was inhibited (Fig. 8O and P). These data indicate that PKM2-mediated mitochondrial protection occurs through PKM2-dependent PHB2 phosphorylation.
PHB2 dephosphorylation abolishes PKM2-mediated mitochondrial protection. HL-1 cardiomyocytes were transfected with PKM2 overexpression adenovirus (Ad-PKM2), a phosphorylation-defective PHB2S91A mutant, or a phosphorylation-mimetic PHB2 S91D mutant before LPS treatment. Adenovirus loaded β-gal (Ad-β-gal) cells were used as controls. (A-C) Immunofluorescence analysis of mitochondrial morphology in HL-1 cells. Representative images of mitochondria immunofluorescence (A), average mitochondrial length (B), and proportion of cardiomyocytes with fragmented mitochondria (C) are shown. (D-J) Western blot analysis of Drp1, Fis1, Mfn2, Opa1, Parkin, and Atg5 in HL-1 cells. (K, L) Mitophagy analysis results (mt-Keima assay). (M-P). Transcriptional analysis of Tfam, Nrf2, mtHsp70, and Atf6 expression by qPCR. Values are presented as mean ± SEM. For in vivo data, n = 6 mice per group. For in vitro data, n = 4 independent experiments. #p < 0.05, and ##p < 0.01
PKM2-mediated cardiomyocyte protection against septic insult requires PHB2 phosphorylation
To illustrate the necessary role of PHB2 phosphorylation in supporting PKM2-induced cardioprotection, HL-1 cardiomyocyte viability and function were determined after transfection with PHB2S91D or PHB2S91A mutants. LPS exposure promoted the release of cardiac injury markers, e.g. TnI, CK-MB, and LDH, into the culture medium in control cells, and this trend was decreased by either PKM2 overexpression or introduction of the phospho-mimetic PHB2S91D mutant (Fig. 9A and C). In line with the findings described above, PKM2 overexpression failed to prevent the upregulation of cardiac damage markers in HL-1 cells co-transfected with the phospho-defective PHB2S91A mutant after LPS challenge (Fig. 9A and C). In turn, both cell viability and survival assessed by CCK-8 assay (Fig. 9D) and TUNEL staining (Fig. 9E and F), respectively, were improved by either PKM2 overexpression or PHB2S91D mutant transfection. In contrast, PKM2 overexpression-mediated cardiomyocyte survival was decreased in cells co-expressing PHB2S91A (Fig. 9D and F). Moreover, LPS-stimulated transcription of pro-inflammatory factors was significantly suppressed by either PKM2 or PHB2S91D overexpression, while the anti-inflammatory action of PKM2 was impaired upon transfection of PHB2S91A (Fig. 9G-H). Finally, either PKM2 or PHB2S91D, but not PHB2S91D transfection, largely preserved myosin filament expression and organization in cardiomyocytes exposed to LPS (Fig. 9I-J). The above evidence thus confirms that PKM2-mediated cardioprotection during septic conditions requires PHB2 phosphorylation.
PKM2-mediated cardiomyocyte protection against septic insult requires PHB2 phosphorylation. HL-1 cardiomyocytes were transfected with PKM2 overexpression Adenovirus (Ad-PKM2), a phosphorylation-defective PHB2S91A mutant, or a phosphorylation-mimetic PHB2 S91D mutant before LPS treatment. Adenovirus loaded β-gal (Ad-β-gal) cells were used as controls. (A, C) ELISA-based analysis of TnI, CK-MB, and LDH levels in culture media of HL-1 cells transfected with phosphorylation-defective (PHB2S91A) and phosphorylation-mimetic (PHB2S91D) mutant constructs. (D) Cell viability analysis via CCK-8 assay in vitro. (E, F) Apoptosis analysis by TUNEL staining in cultured HL-1 cells. (G, H) Transcriptional analysis of Tnfα and Mcp1 expression. (I, J) Representative images of myosin immunofluorescence. Myosin expression levels were normalized to those of the control group. Values are presented as mean ± SEM. For in vivo data, n = 6 mice per group. For in vitro data, n = 4 independent experiments. #p < 0.05, and ##p < 0.01
Knockin mice expressing phospho-mimetic Phb2 S91D are less vulnerable to SC
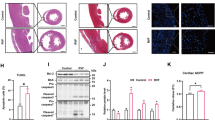
To investigate whether PKM2-mediated PHB2 phosphorylation confers myocardial protection against SC in vivo, transgenic knockin mice carrying the Phb2S91D variant were generated. Total-PHB2 was used as the loading control to analyze the changes in p-PHB2S91. In comparison to WT mice, heart tissues from homozygous Phb2S91D/D mice showed elevated levels of p-PHB2S91 (Fig. 10A-B). Since PHB2 phosphorylation prevents PHB2 degradation, we further examined the alterations in total-PHB2 expression in WT, heterozygous Phb2S91D/+, and homozygous Phb2S91D/D mice. After normalizing to α-tubulin, the levels of total-PHB2 were significantly reduced in LPS-treated WT mice (Fig. 10C-D). However, these changes were partially prevented in Phb2S91D/+ mice and fully restored in Phb2S91D/D. Furthermore, the mtDNA/nDNA ratio was employed to assess potential alterations in mitochondrial content or mitochondrial copy numbers in transgenic knockin mice harboring the Phb2S91D variant. As depicted in Fig. 10E, the mtDNA/nDNA ratio exhibited no significant changes among WT, heterozygous Phb2S91D/+, and homozygous Phb2S91D/D mice.
Knockin mice expressing phospho-mimetic Phb2S91 are less vulnerable to SC. Heterozygous Phb2S91D/+ mice, homozygous Phb2S91D/D mice, and wild-type (WT) mice aged 8–10 weeks were injected intraperitoneally with 10 mg/kg LPS to induce SC. Mice administered an equal volume of phosphate buffer saline served as controls. (A, B) Western blot analysis of cardiac p-PHB2S91 in WT, heterozygous Phb2S91D/+, and homozygous Phb2S91D/D mice treated with PBS or LPS. Total-PHB2 was used as the loading control. (C, D) Western blot analysis of total-PHB2 in WT, heterozygous Phb2S91D/+, and homozygous Phb2S91D/D mice treated with PBS or LPS. α-tubulin was used as the loading control. (E) The CO1 gene of mtDNA and the NDUFV1 gene of nDNA were amplified using qPCR to assess the relative ratio of mtDNA/nDNA in heart tissues obtained from WT, heterozygous Phb2S91D/+, and homozygous Phb2S91D/D mice under normal physiological conditions. (F-L) Echocardiographic evaluation. LVDd, left ventricular diastolic dimension; LVDs, left ventricular systolic dimension; IVS, interventricular septum thickness; E/A, ratio of early to late transmitral flow velocities; FS, ratio of left ventricular fractional shortening. (M-O) ELISA-based analysis of serum TnI, CK-MB, and LDH concentrations. (P-R) Transcriptional analysis of cardiac Mmp9, Mcp1, and Tnfα expression by qPCR. (S) Representative images of TnT and Gr-1 immunohistochemistry in cardiac sections. (T) Quantification of Gr-1-positive neutrophils in mouse heart tissues. (U) ELISA-based measurement of caspase-3 activity in heart tissues. Values are presented as mean ± SEM. For in vivo data, n = 6 mice per group. For in vitro data, n = 4 independent experiments. #p < 0.05, and ##p < 0.01
Heart function, assessed by echocardiography, was slightly improved in Phb2S91D/+ mice (Fig. 10F and L), and largely normalized in Phb2S91D/D mice upon LPS-induced SC. Similarly, LPS-stimulated TnI, CK-MB, and LDH were partially reduced in Phb2S91D/+ mice and significantly inhibited in Phb2S91D/D mice (Fig. 10M and O). Along with these changes, LPS-induced transcription of Mmp9, Mcp1, and Tnfα was downregulated in Phb2S91D/+ mice, and further repressed in Phb2S91D/D mice (Fig. 10P and R). In accordance with these alterations, myocardial accumulation of Gr-1 positive neutrophils was relived in Phb2S91D/+ mice and completely prevented in Phb2S91D/D mice (Fig. 10S and T). Lastly, LPS-induced cardiomyocyte death, as assessed by caspase-3 activity, was partly attenuated in Phb2S91D/+ mice and remarkably inhibited in Phb2S91D/D mice (Fig. 10U). Taken together, our results elucidated that PHB2 phosphorylation mediates myocardial protection against LPS-induced SC.