Abstract
Background
Hypereosinophilic dermatitis (HED) is a subtype of hypereosinophilic syndrome (HES). Glucocorticoids are preferred for treatment but carry substantial side effect profiles. Symptoms of HED may recur after systemic glucocorticoid tapering. As an interleukin-4 receptor (IL-4Rα) monoclonal antibody targeting interleukin-4 (IL-4) and interleukin-13 (IL-13), dupilumab might be an efficacious adjuvant therapy for HED.
Method
We report a young male diagnosed with HED who suffered from erythematous papules with pruritus for over five years. Once reducing the dosage of glucocorticoid was, his skin lesions relapsed.
Results
After using dupilumab, the patient’s condition significantly improved with the glucocorticoid dosing decreased successfully.
Conclusion
In conclusion, we report a new application of dupilumab in HED patients, especially with difficulties in reducing the glucocorticoid dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hypereosinophilic syndrome (HES) is a group of rare disorders characterized by eosinophilia and eosinophil activation with damage to multiple organ systems. The clinical diagnostic criteria for HES include a sustained peripheral blood absolute eosinophil count greater than 1.5 × 109/L for more than 6 months or pathologic confirmation of tissue hypereosinophilia in the absence of an identifiable cause [1, 2]. Hypereosinophilic dermatitis (HED), a subtype of HES, generally presents infiltrative skin erythema, plaques, intense pruritus, and increased peripheral blood eosinophilia. Currently, systemic glucocorticoids and immunosuppressants are commonly used to treat HED [3]. However, long-term use of high-dose glucocorticoids has several adverse consequences, including hypertension, obesity, diabetes, and osteoporosis. Biologics that reduce eosinophilic inflammation directly or indirectly provide an alternative treatment option [4].
Dupilumab is a humanized monoclonal antibody targeting the interleukin‐4 receptor α (IL-4Rα) that acts on both interleukin-4 (IL-4) and interleukin-13 (IL-13)-related signaling pathways to inhibit T helper 2 (Th2) responses. Dupilumab has been approved for moderate-to-severe atopic dermatitis and eosinophilic asthma. It also showed efficacy in several cases of bullous pemphigoid, including most recalcitrant cases, such as those failing treatment with rituximab [5]. The multicentre phase II and III clinical trials have currently been undergone in bullous pemphigoid patients (NCT04206553, NCT05649579, and NCT04776694). Eosinophils have long been accepted as the hallmark of Th2-type immune responses and could secrete cytokines, such as IL-4 and interleukin-5 (IL-5) [6, 7]. Peripheral blood eosinophils in patients with atopic dermatitis were decreased after using dupilumab. We report a case of a young male with refractory HED whose symptoms showed improvement and glucocorticoid dosing successfully tapering by dupilumab.
Case report
A 35-year-old male presented to our department for a 4 year history of severely erythematous papules with pruritus distributed over his trunk and limbs with no obvious precipitating. He was misdiagnosed with papular urticaria, eczema, and atopic dermatitis, leading to poor treatment efficacy. At the time of his initial visit to our dermatology clinic, the blood eosinophil count was 1.33 × 109/L (normal range 0.02 ~ 0.52 × 109/L), the total immunoglobulin E (IgE) was 114 IU/ml (normal range < 165 IU/ml), and the allergens test showed no abnormalities. He took oral systemic glucocorticoids (methylprednisolone), antihistamines, and traditional Chinese medicine, combined with topical medication and intermittent intramuscular injections of betamethasone. His condition was relatively relieved using glucocorticoids, but after the reduction of the glucocorticoid dose, his symptoms recurred and aggravated over time.
On admission, his routine examinations suggested a blood eosinophil count of 5.14 × 109/L. The antinuclear antibodies and complement were normal, but an increased level of IgE was observed. The stool microscopy showed parasite-negative and worm-negative. A skin biopsy was carried out, which showed a large number of eosinophils infiltration distributed around the blood vessels and collagen bundles, without subepidermal detachment, subepidermal bulla, or avascular necrosis (Fig. 1a, b). His bone marrow biopsy also showed increased eosinophils (25%). These pathological results were inconsistent with diagnostic criteria for atopic dermatitis, bullous pemphigoid, recurrent cutaneous necrotizing eosinophilic vasculitis (RCNEV), and Wells’ syndrome. Thus, a final diagnosis of eosinophilic dermatitis was made.
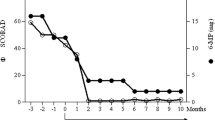
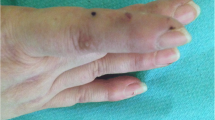
Histopathological photomicrographs, clinical photographs, timelines of drug used, and IgE levels of the patient. a–b Histopathological image of the lesions showed abundant eosinophils infiltration (red arrows—eosinophils; red circle—flame figure). c The timelines of methylprednisolone daily doses and IgE levels during the treatment period (red arrow—the time of dupilumab first application). d–e The clinical photographs taken before the Dupilumab application, after 2 months, and after 3 months of admission (left to right)
He had been taking glucocorticoids for many years. The patient’s symptoms recurred when methylprednisolone was reduced to 16 mg though combined with mycophenolate mofetil. Due to the long-term use of glucocorticoids, he experienced side effects, such as Cushing’s syndrome, gastroesophageal reflux, and osteopenia. He lost confidence and thought that the disease seriously affected his quality of life. Therefore, 1 year ago, after informing and understanding the drug mechanism and potential risks of dupilumab, he accepted this biologic agent as adjuvant therapy to assist himself in glucocorticoid reduction.
After using dupilumab for 1 year (600 mg subcutaneously initially and 300 mg subcutaneously every other week), the glucocorticoid dose decreased with adequate skin lesions control with the IgE serum levels reducing (Fig. 1c). He used methylprednisolone 2 mg daily with dupilumab 300 mg every other week for long-term treatment. Because of the COVID-19 vaccine injection, he had to stop taking methylprednisolone for 2 weeks. Pleasingly, the patient did not develop any new papules following withdrawal. His Cushing's symptoms significantly improved, as did the patient’s quality of life, and no adverse effects were observed (Fig. 1d, e).
Discussion
As is well known, for diseases treated with systemic glucocorticoid, medication tapering is an essential clinical conundrum that requires consideration in addition to the ability to control the disease. Long-term use of glucocorticoids not only affects patients’ appearance (Cushing’s syndrome including full moon face, buffalo back, and central obesity) but also causes blood glucose increments, osteoporosis, and secondary infections. Currently, glucocorticoid tapering is usually supplemented by combined immunosuppressive agents, but side effects like secondary infections also accompany the use of immunosuppressive agents.
HED is a rare heterogeneous disease characterized by the presence of diverse skin lesions and peripheral blood eosinophilia, which may be accompanied by elevated IgE [8]. The efficacy of biologic agents for the treatment of HES suggests that type 2 inflammation can be an underlying factor beyond eosinophilic inflammation in disease manifestations in some patients [4]. Dupilumab is a blockade of IL-4 and IL-13, and two of the main Th2 effector cytokines play essential roles in the downstream Th2 reaction. It is also a potential treatment option for eosinophil-mediated dermatoses like bullous pemphigoid. This case describes a patient with HED of unknown cause, whose erythematous papules recurred after tapering glucocorticoid treatment. After 1 year of treatment with dupilumab, the patient’s symptoms improved, and the glucocorticoid consumption decreased successfully. Levels of IgE also decreased after treatment. The glucocorticoid side effects decreased significantly and the patient’s health-related quality of life improved. Although the price for dupilumab is high, the patient was delighted because of the efficacy results.
Several cases of dupilumab application in eosinophil-associated diseases have been published [9, 10]. This case adds more evidence for using dupilumab in HED and showed that dupilumab could be used as a neoadjuvant for Th2 inflammation-related diseases, especially if there are difficulties in reducing the glucocorticoid dose. The successful treatment of this patient demonstrates the wide range of uses of dupilumab as a biologic agent. However, clinical trials are needed to confirm these preliminary observations.
Data availability
The authors declare that all data supporting the findings of this study are available within the article.
References
Valent P, Klion AD, Roufosse F, Simon D, Metzgeroth G, Leiferman KM, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2022. https://doi.org/10.1111/all.15544.
Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. https://doi.org/10.1038/nri3341.
Requena G, van den Bosch J, Akuthota P, Kovalszki A, Steinfeld J, Kwon N, et al. Clinical profile and treatment in hypereosinophilic syndrome variants: a pragmatic review. J Allergy Clin Immunol. 2022;10:2125–34. https://doi.org/10.1016/j.jaip.2022.03.034.
Chen MM, Roufosse F, Wang SA, Verstovsek S, Durrani SR, Rothenberg ME, et al. An international, retrospective study of off-label biologic use in the treatment of hypereosinophilic syndromes. The J Allergy Clin Immunol. 2022;10:1217-1228.e3. https://doi.org/10.1016/j.jaip.2022.02.006.
Baffa ME, Maglie R, Montefusco F, Pipitò C, Senatore S, Antiga E. Severe bullous pemphigoid following Covid-19 vaccination resistant to rituximab and successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023. https://doi.org/10.1111/jdv.18673.
Satoh T, Yokozeki H, Nishioka K. Pathogenic roles of eosinophils in guinea-pig contact sensitivity: regulation of dermal eosinophilia with remotely administered IL-5. Clin Exp Immunol. 2000;122:300–7. https://doi.org/10.1046/j.1365-2249.2000.01355.x.
Franke K, Notaro E, Moshiri AS, Ayars A, Kalus A. Use of Dupilumab and eosinophil targeted therapy in treating angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2022;158:960–2. https://doi.org/10.1001/jamadermatol.2022.1969.
Oh JE, Oh DS, Jung HE, Lee HK. A mechanism for the induction of type 2 immune responses by a protease allergen in the genital tract. Proc Nat Acad Sci. 2017. https://doi.org/10.1073/pnas.1612997114.
Maglie R, Ugolini F, De Logu F, Simi S, Senatore S, Montefusco F, et al. Dupilumab for the treatment of recalcitrant eosinophilic dermatosis of haematologic malignancy. J Eur Acad Dermatol Venereol. 2021. https://doi.org/10.1111/jdv.17232.
Goyal A, Lofgreen S, Mariash E, Bershow A, Gaddis KJ. Targeted inhibition of IL-4/13 with dupilumab is an effective treatment for eosinophilic dermatosis of hematologic malignancy. Dermatol Therapy. 2020. https://doi.org/10.1111/dth.13725.
Funding
This work was supported by grants from the Medical Health Science and Technology Project of Hangzhou, China (ZD20220089).
Author information
Authors and Affiliations
Contributions
XJ and SC contributed to the manuscript draft, patient management, and research on the topic. JY, XW, JZ, and HC contributed to the patient management and revised the manuscript. HC supervised the study, and revised the final version of the manuscript and responsible for supervision and funding acquisition. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest or competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by the Sir Run Run Shaw Hospital Ethical Committee. The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, X., Ye, J., Wu, X. et al. A case of complete recovery in a hypereosinophilic dermatitis patient with dupilumab. Inflamm. Res. 72, 875–878 (2023). https://doi.org/10.1007/s00011-023-01715-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01715-1