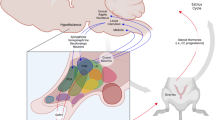
Abstract
One of the more fascinating developments in neuroscience is the recognition of endocrine influences on brain regions unrelated to reproductive and basic homeostatic functions. It is now clear that hormones impact both normal function and dysfunction, including the experience of pleasure and the anhedonia accompanying a number of psychiatric disorders, most notably depression. Brain regions contributing to these functions are rich in receptors for the peptides and steroids of the hypothalamic – pituitary – gonadal (HPG) and hypothalamic – pituitary – adrenal (HPA) axes. Indeed, the brain has evolved new functions for ancient hormones. Examples include the brain adaptive uses of steroid precursors and metabolites for non-reproductive functions and the brain co-opting or “hijacking” peptides of the two axes to serve as neuromodulators and neurotransmitters. The result is that HPA and HPG hormones and their interactions have profound influences on opioids and monoamines, especially dopamine and serotonin. These are the same neurotransmitter pathways underlying activation of the brain reward pathway stretching from midbrain to the prefrontal cortex.
Our ultimate goal is to fulfill the promise of the title, an evaluation of neuroendocrine – anhedonia relations. This requires, first, an overview of the endocrine system, and their steroids and peptides. There, we also provide a brief review of the interaction of the HPA and HPG axes in depression. Before embarking on an evaluation of hormones and anhedonia, we will examine normal neuroendocrine influences on pleasure from natural experiences such as food and sex but also from psychoactive drugs. Logic suggests examining data on pleasure before addressing loss of pleasure. The emphasis throughout will be on animal models with a liberal sprinkling of human findings, mostly psychiatric patients.
This journey will take us through endocrine basics (Sect. 10.1), and the influence of hormones on brain systems underlying the experience of pleasure (Sect. 10.2). In Sect. 10.3, the modest literature on the neuroendocrinology of anhedonia in depression will be reviewed. Finally, future research and directions (Sect. 10.4) will provide ideas on filling in the gaps in our understanding of endocrine – anhedonia relations.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 5HT:
-
Serotonin
- ACTH:
-
Adrenocorticotropin hormone
- ALLO:
-
Allopregnanolone
- AVP:
-
Arginine vasopressin
- BNST:
-
Bed nucleus of the stria terminalis
- BRS:
-
Brain reward system
- CMS:
-
Chronic mild stress
- CORT:
-
Corticosteroid
- CRH:
-
Corticotropin-releasing hormone
- CSF:
-
Cerebrospinal fluid
- DA:
-
Dopamine
- DHEA:
-
Dehydroepiandrosterone
- DOPAC:
-
3, 4-Dihydroxyphenlacetic acid
- E2:
-
Estradiol
- EPI:
-
Epinephrine
- FST:
-
Forced swim test
- GABA:
-
Gamma-Aminobutyric acid
- GnRH:
-
Gonadotropin releasing hormone
- GR:
-
Glucocorticoid receptor
- HPA:
-
Hypothalamic-pituitary-adrenal
- HPG:
-
Hypothalamic-pituitary-gonadal
- HVA:
-
Homovanillic acid
- ICSS:
-
Intracranial self-stimulation
- LH:
-
Luteinizing hormone
- MDD:
-
Major Depressive Disorder
- MFB:
-
Medial forebrain bundle
- MR:
-
Mineralocorticoid receptor
- NAcc:
-
Nucleus accumbens
- NE:
-
Norepinephrine
- OVX:
-
Ovariectomy
- PFC:
-
Prefrontal cortex
- POMC:
-
Proopiomelanocortin
- PROG:
-
Progesterone
- PVN:
-
Paraventricular nucleus of the hypothalamus
- SAM:
-
Sympathetic adrenal medullary system
- SSRI:
-
Selective serotonin reuptake inhibitors
- TH:
-
Tyrosine hydroxylase
- TS:
-
Testosterone
- VTA:
-
Ventral tegmental area
References
Ruedi-Bettschen D, Zhang W, Russig H, et al. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. Eur J Neurosci. 2006;24:2879–93.
Frodl T, Reinhold E, Koutsouleris N, et al. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010;44:799–807.
McEwen BS, Eiland L, Hunter RG, et al. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12.
Heim C, Owens MJ, Plotsky PM, et al. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull. 1997;33:185–92.
Pohl J, Olmstead MC, Wynne-Edwards KE, et al. Repeated exposure to stress across the childhood–adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–74.
de Kloet ER. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–300.
Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions an introduction. Ann N Y Acad Sci. 2004;1024:1–8.
Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci. 2009;1179:41–55.
de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–76.
Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313.
Stern CM. Corticotropin-releasing factor in the hippocampus: eustress or distress? J Neurosci. 2011;31:1935–6.
Sgoifo A, DeBoer SF, Haller J, et al. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: relationship with aggression. Physiol Behav. 1996;60:1403–7.
Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–84.
Sapolsky RM. Stress, the aging brain, and the mechanisms of neuronal death. Cambridge, MA: The M.I.T. Press; 1992.
Taylor G, Bardgett M, Csernansky J, et al. Male rat reproductive systems under chronic fluoxetine or trimipramine treatment. Physiol Behav. 1996;59:479–85.
Taylor GT, Weiss J, Zimmermann F. Animal models of sex differences in nonreproductive brain function. In: Tatlisumak T, Fisher M, editors. Handbook of experimental neurology: methods and techniques in animal research. New York: Cambridge University Press; 2006. p. 239–56.
Conneely OM, Mulac-Jericevic B, DeMayo F, et al. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55.
Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32.
Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–70.
Brunton PJ, Russell JA. Allopregnanolone and suppressed hypothalamo-pituitary-adrenal axis stress responses in late pregnancy in the rat. Stress. 2011;14:6–12.
Kessler RC. Gender differences in major depression: epidemiologlcal findings. In: Frank E, editor. Gender and its effects on psychopathology. Washington, DC: American Psychiatric Press; 2000. p. 61–84.
Taylor GT, Boggiano J, Cabrera O, et al. Steroidal influences on anxiety disorders in childhood and their animal models. Curr Top Steroid Res. 2011;8:47–64.
Gonda X, Telek T, Juhasz G, et al. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1782–8.
Bloch M, Daly RC, Rubinov DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–46.
Meltzer-Brody S. Understanding and treating mood disorders across the reproductive years. Sex Reprod Menopause. 2010;8:12–8.
Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109.
Chiba S, Numakawa T, Ninomiya M, et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:112–9.
Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32.
Post RM, Weiss SR, Li H, et al. Neural plasticity and emotional memory. Dev Psychopathol. 1998;10:829–55.
Wardenaar KJ, Vreeburg SA, van Veen T, et al. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biol Psychiatry. 2011;69:366–73.
Barden N. Implication of the hypothalamic – pituitary – adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–93.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501.
Romer B, Lewicka S, Kopf D, et al. Cortisol metabolism in depressed patients and healthy controls. Neuroendocrinology. 2009;90:301–6.
Wolkowitz OM, Burke H, Epel ES, et al. Mood, memory, and mechanisms. Ann N Y Acad Sci. 2009;1179:19–40.
Hoschl C, Hajek T. Hippocampal damage mediated by corticosteroids–a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 2:II81–8.
DeSouza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819.
Duncko R, Kiss A, Skultetyova I, et al. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89.
Muller MB, Keck ME. Genetically engineered mice for studies of stress-related clinical conditions. J Psychiatr Res. 2002;36:53–76.
Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol. 1991;38:537–58.
Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–6.
Krugers HJ, Lucassen PJ, Karst H, et al. Chronic stress effects on hippocampal structure and synaptic function: relevance for depression and normalization by anti-glucocorticoid treatment. Front Synaptic Neurosci. 2010;2:24.
Kubera M, Obuchowicz E, Goehler L, et al. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:744–59.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–65.
Sheline YI, Sanghav M, Mintun M, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43.
Olney JW. Excitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol. 1990;30:47–71.
Kaminska M, Harris J, Gijsbers K, et al. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP: implications for depression. Brain Res Bull. 2000;52:229–34.
Naert G, Maurice T, Tapia-Arancibia L, et al. Neuroactive steroids modulate HPA axis activity and cerebral brain derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32:1062–78.
Maninger N, Wolkowitz OM, Reus VI, et al. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Endocrinol. 2009;30:65–91.
Herbert J. Neurosteroids, brain damage, and mental illness. Exp Gerontol. 1998;33:713–27.
Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32.
Matsumoto AM, Bremner WJ. Serum testosterone assays–accuracy matters. J Clin Endocrinol Metab. 2004;89:520–4.
Becker JB, Monteggia LM, Perrot-Sinal TS, et al. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5.
Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–76.
McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33.
Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52:318–27.
Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–8.
Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–8.
McCormick CM, Linkroum W, Sallinen BJ, et al. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47.
Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:766–76.
Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–33.
Yang S-J, Kim S-Y, Stewart RB, et al. Gender differences in 12-week antidepressant treatment outcomes for a naturalistic secondary care cohort: the CRESCEND study. Psychiatry Res. 2011;189:82–90.
Baca E, Garcia-Garcia M, Porras-Chavarinoc A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:57–65.
Kornstein SG, Sloan DME, Thase ME. Gender-specific differences in depression and treatment response. Psychopharmacol Bull. 2002;36 Suppl 3:99–112.
Osterlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800:1136–44.
Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor α messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–42.
Young EA, Midgley AR, Carlson NE, et al. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57:1157–62.
Rocca WA, Grossardt BR, Geda YE, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–9.
Holsen LM, Spaeth SB, Lee JH, et al. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131:379–87.
Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–8.
Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83.
Baischer W, Koinig G, Hartmann B, et al. Hypothalamic–pituitary–gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–9.
Hardoy MC, Serra M, Carta MG, et al. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. 2006;26:379–84.
Nin MS, Martinez LA, Pibiri F, et al. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol. 2011;2:73. doi:10.3389/fendo.2011.00073.
Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30.
Martin-Soelch C. Is depression associated with dysfunction of the central reward system? Biochem Soc Trans. 2009;37:313–7.
Nestler EJ, Carlezon JWA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Skinner BF. The behavior of organisms. New York: Appleton; 1938.
Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25:235–51.
Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–41.
Russell VA. Dopamine hypofunction possibly results from a defect in glutamate-stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder–the spontaneously hypertensive rat. Neurosci Biobehav Rev. 2003;27:671–82.
Parolaro D, Realini N, Vigano D, et al. The endocannabinoid system and psychiatric disorders. Exp Neurol. 2010;224:3–14.
Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11.
DiChiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–33.
Pfaus JG, Damsma G, Wenkstern D, et al. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30.
Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm Behav. 2003;44:419–28.
Putnam SK, Sato S, Riolo JV, et al. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm Behav. 2005;47:513–22.
Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:435–51.
Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115.
Andersen ML, Sawyer EK, Howell LL. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol. 2012;20:2–15.
Aron A, Fisher HE, Mashek D, et al. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37.
Levita L, Hare TA, Voss HU, et al. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–87.
Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–86.
Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:303–10.
Salamone J, Cousins M, Snyder B. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–59.
Rademacher L, Krach S, Kohls G, et al. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–85.
Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–26.
Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41.
Becker JB. Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009;55:646–54.
Volkow ND, Wang G-J, Fischman MW, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–30.
Everitt BJ. Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses in male rats. Neurosci Biobehav Rev. 1990;14:217–32.
Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209.
Colasanti A, Searle GE, Long CJ, et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72:371–7.
Nakahara D, Nakamura M, Oki Y, et al. Lack of glucocorticoids attenuates the self-stimulation-induced increase in the in vivo synthesis rate of dopamine but not serotonin in the rat nucleus accumbens. Eur J Neurosci. 2000;12:1495–500.
Barrot M, Marinelli M, Abrous DN, et al. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–80.
Lindley SE, Tasha G, Bengoechea TG, et al. Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology. 1999;21:399–407.
Mizoguchi K, Ishige A, Takeda S, et al. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–9.
Chocyk A, Dudys D, Przyborowska A, et al. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. 2011;173:1–18.
Cabib S, Puglisi-Allegra S, Damato F. Effects of postnatal stress on dopamine mesolimbic system responses to aversive experiences in adult life. Brain Res. 1993;604:232–9.
Cabib S, D’Amato FR, Puglisi-Allegra S, et al. Behavioral and mesocorticolimbic dopamine responses to non aggressive social interactions depend on previous social experiences and on the opponent’s sex. Behav Brain Res. 2000;112:13–22.
Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–28.
Cabib S, Puglisi-Allegra S. Different effects of repeated stress on mesocortical and mesolimbic dopamine metabolism. Neuroscience. 1996;73:375–80.
Dziedzicka-Wasylewska M, Willner P, Papp M. Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol. 1997;8:607–18.
Koob GF, Caine SB, Parsons L, et al. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–21.
Boutrel B. A neuropeptide-centric view of psychostimulant addiction. Br J Pharmacol. 2008;154:343–57.
Elman I, Lukas SE, Karlsgodt KH, et al. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–9.
Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94.
Goodman A. Neurobiology of addition: an integrative review. Biochem Pharmacol. 2008;75:266–322.
Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–98.
Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–12.
Dellu F, Mayo W, Vallee M, et al. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly – a life-span study of rats. Psychoneuroendocrinology. 1996;21:441–53.
Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8.
Hooks MS, Jones GH, Smith AD, et al. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–8.
Piazza P, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74.
Dluzen DE, Salvaterra TJ. Sex differences in methamphetamine-evoked striatal dopamine output are abolished following gonadectomy: comparisons with potassium-evoked output and responses in prepubertal mice. Neuroendocrinology. 2005;82:78–86.
**ao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–8.
Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–27.
Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86:354–67.
Yague JG, Wang AC, Janssen WG, et al. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–27.
Shieh KR, Yang SC. Effects of estradiol on the stimulation of dopamine turnover in mesolimbic and nigrostriatal systems by cocaine- and amphetamine-regulated transcript peptide in female rats. Neuroscience. 2008;154:1589–97.
McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307.
Russo SJ, Festa ED, Fabian SJ, et al. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–33.
Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine: implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:173–87.
Izumo N, Ishibashi Y, Ohba M, et al. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav Brain Res. 2012;227:1–6.
DiPaolo T, Rouillard C, Bedard P. 17B-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203.
Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–41.
Morris ME, Lee HJ, Predko LM. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol Rev. 2003;55:220–40.
Shansky RM, Glavis-Bloom C, Lerman D, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–8.
Dazzi L, Seu E, Cherchi G, et al. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901.
Jacome LF, Gautreaux C, Inagaki T, et al. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–98.
Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-ß-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13:282–96.
Sanchez MG, Bourque M, Morissette M, et al. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e43–71.
Maggi M, Ciana P, Belcredito S, et al. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu Rev Physiol. 2004;66:291–313.
Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22.
van der Staay FJ. Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res Rev. 2006;52:131–59.
Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57.
Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–82.
Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–14.
Von Frijtag JC, Reijmers LG, Van der Harst JE, et al. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–46.
Miczek KA, Nikulina EM, Takahashi A, et al. Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet. 2011;41:787–802.
Razzoli M, Carboni L, Arban R. Alterations of behavioral and endocrinological reactivity induced by 3 brief social defeats in rats: relevance to human psychopathology. Psychoneuroendocrinology. 2009;34:1405–16.
Taylor GT, Smith SE, Kirchhoff BA. Differential effects of antipsychotics on lateral bias and social attention in rats. Psychopharmacology. 2013;225:453–60.
Hill MN, Hellemans KG, Verma P, et al. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–117.
Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29.
Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43.
Dang H, Chen Y, Liu X, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1417–24.
Pan Y, Wang FM, Qiang LQ, et al. Icariin attenuates chronic mild stress-induced dysregulation of the LHPA stress circuit in rats. Psychoneuroendocrinology. 2010;35:272–83.
Grippo AJ, Francis J, Beltz TG, et al. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706.
Grippo AJ, Sullivan NR, Damjanoska KJ, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl). 2005;179:769–80.
Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev. 2009;33:1089–98.
Konkle ATM, Baker SL, Kentner AC, et al. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–38.
Dalla C, Antoniou K, Drossopoulou G, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–14.
Bachis A, Cruz MI, Nosheny RL, et al. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008;442:104–8.
Garcia LS, Comim CM, Valvassori SS, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:450455.
Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354(1):155–69. doi:10.1007/s00441-013-1664-0.
Elizalde N, Garcia-Garcia AL, Totterdell S, et al. Sustained stress-induced changes in mice as a model for chronic depression. Psychopharmacology. 2010;210:393–406.
Muscat R, Papp M, Willner P. Reversal of stress – induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology. 1993;109:433–8.
Grippo AJ, Beltz TG, Weiss RM, et al. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–16.
Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–54.
Troisi A, Alcini S, Coviello M, et al. Adult attachment style and social anhedonia in healthy volunteers. Personal Individ Differ. 2010;48:640–3.
Bennett DS, Ambrosini PJ, Kudes D, et al. Gender differences in adolescent depression: do symptoms differ for boys and girls? J Affect Disord. 2005;89:35–44.
Bielajew C, Konkle AT, Kentner AC, et al. Strain and gender specific effects in the forced swim test: effects of previous stress exposure. Stress. 2003;6:269–80.
D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–7.
Benelli A, Filaferro M, Bertolini A, et al. Influence of Sadenosyl-L-methionine on chronic mild stress-induced anhedonia in castrated rats. Br J Pharmacol. 1999;127:645–54.
Pitychoutis PM, Dalla C, Sideris AC, et al. 5-HT1A, 5-HT2A, and 5-HT2C receptor mRNA modulation by antidepressant treatment in the chronic mild stress model of depression: sex differences exposed. Neuroscience. 2012;210:152–67.
Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, et al. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–22.
Gronli J, Murison R, Fiske E, et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–7.
Herrera-Perez JJ, Martinez-Mota L, Chavira R, et al. Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Horm Behav. 2012;61:623–30.
Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry. 2012;71:642–51.
Hellemans KG, Verma P, Yoon E, et al. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–75.
Bloch M, Schmidt PJ, Danaceau MA, et al. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45:1533–41.
Harden M, Smith SE, Niehoff JA, et al. Anti-depressive effects of the κ-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behav Pharmacol. 2012;23:710–5.
Recamier-Carballo S, Estrada-Camarena E, Reyes R, et al. Synergistic effect of estradiol and fluoxetine in young adult and middle-aged female rats in two models of experimental depression. Behav Brain Res. 2012;233:351–8.
Taylor GT, Farr S, Klinga K, et al. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-restored ovariectomized female rats. Psychoneuroendocrinology. 2004;29:1241–9.
Romano-Torres M, Fernandez-Guasti A. Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress. Behav Pharmacol. 2010;21:104–11.
Schiller CE, O’Hara MW, Rubinow DR, et al. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–44.
Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56.
Galankin T, Shekunova E, Zvartau E. Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav. 2010;58:827–34.
Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2010;58:22–32.
Auriacombe M, Reneric JP, LeMoal M. Animal models of anhedonia. Psychopharmacology. 1997;134:337–8.
Weiss JM, Kilts CD. Animal models of depression and schizophrenia. In: Schatzberg A, Nemeroff C, editors. Textbook of psychopharmacology. 2nd ed. Washington, DC: American Psychiatric Press; 1998. p. 89–131.
Morley JE, Kim MJ, Haren MT. Frailty and hormones. Rev Endocr Metab Disord. 2005;6:101–8.
Lam RW. Onset, time course and trajectories of improvement with antidepressants. Eur Neuropsychopharmacol. 2012;22 Suppl 3:S492–8.
Taylor GT, Weiss J, Rupich R. Male rat behavior, endocrinology and reproductive physiology in a mixed-sex, socially stressful colony. Physiol Behav. 1987;39:429–33.
Altemus M. Hormone-specific psychiatric disorders: do they exist? Arch Womens Ment Health. 2010;13:25–6.
Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126:73–85.
Acknowledgments
Preparation of this manuscript was supported in part by grants from the University of Missouri Research Board and the College Dean’s Faculty Research Awards at UM-St. Louis. The authors thank Joseph Boggiano, now at the Washington University School of Medicine in St. Louis, Missouri, for assistance in the conduct of the unpublished experiment cited in the manuscript. Also, we thank Dr. Juergen Weiss of the University of Heidelberg (Germany) for assistance in preparing the figure (Fig. 10.1) depicting the metabolic cascade in the brain.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Taylor, G.T., Cabrera, O., Hoffman, J. (2014). The Neuroendocrinology of Anhedonia. In: Ritsner, M. (eds) Anhedonia: A Comprehensive Handbook Volume I. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8591-4_10
Download citation
DOI: https://doi.org/10.1007/978-94-017-8591-4_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8590-7
Online ISBN: 978-94-017-8591-4
eBook Packages: MedicineMedicine (R0)