Abstract
Post-attachment resistance can be verified by observing the infection and growth rates of Striga attached to host roots. These protocols explain how to infect Striga hermonthica on host roots using rhizotron chambers, a semi-in vitro system that allows one to observe Striga infection without disturbing the host's roots. Sectioning parasitic attachments and subsequent histological methods are also described, which allow closer investigations of the extent to which the Striga haustoria penetrate host tissues. Such observations can reveal whether any mechanical resistance exists in the host. These histological methods help further characterize resistance mechanisms in rice or sorghum.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Striga resistance can be classified into two types, pre-attachment and post-attachment. As Striga parasitism advances on its cereal hosts, pre-attachment processes include germination and haustorium formation, whereas post-attachment processes include penetration, reaching the host stele, xylem connection, and nutrient transfer. To characterize any gained Striga-resistance in mutagenized hosts, it is essential to quantify and qualify the resistance. For qualification of Striga parasitic success, develo** a system in which multiple Striga synchronously infect host roots with minimal influence from environmental conditions are key. For this purpose, we use a rhizotron system, in which host roots infected by Striga are easily observed without disturbing root architecture (Gurney et al. 2006; Yoshida and Shirasu 2009). The rates of infection are quantified with low-power stereo microscopy or magnification lenses at different days of co-culture. When a Striga seedling has more than three pairs of leaves, the vascular connection generally is established (Yoshida and Shirasu 2009). To characterize the resistance mechanisms in detail, histological analysis is useful. The xylem bridge can be observed with simple Safranin O staining, and the cellular level interaction can be analyzed by making thin sections from Technovit 7100-embedded samples. The staining process may then reveal some post-attachment resistance mechanisms (Yoshida and Shirasu 2009), including incompatibility after vascular connection, endodermis blockage, or mechanical barriers to penetration at the root cortex.
Protocols
Rhizotron system for observing Striga infection. Although the rhizotron method is described for rice, it is also suitable for use with a sorghum host. If using sorghum, follow the surface sterilization and germination protocol described in Chapter “An Agar-Based Method for Determining Mechanisms of Striga Resistance in Sorghum”.
Surface sterilize rice seeds by soaking in a tube with 10% commercial bleach (0.525% NaOCl) for 15 min, gently vortexing periodically. Rinse five times with sterile water and then sow on a Whatman GF/A filter paper in a 90 mm petri dish filled with 15–20 ml sterile water. Germinate the seeds and grow for one week in a growth chamber at 26 °C with the 16 h light/8 h dark photoperiod.
Assemble the rhizotron by removing a 2 cm square notch on the shorter side of a square petri dish and its lid using a pair of pliers or a soldering iron. If using a soldering iron, work under a fume hood to remove fumes from melting plastic (Fig. 1). Make two drain holes on the opposite short side of the rectangular petri dish using the soldering iron (Fig. 1). Cut the rockwool and nylon mesh to the same dimensions of the bottom plate of the petri dish. Immerse the rockwool in a tray filled with tap water. Place the rockwool into the petri dish, and cover with nylon mesh. Place the rice seedling on the nylon mesh. Close the petri dish with its lid and secure it with two rubber bands. Place the petri dish vertically on a tray with water, cover the root parts with aluminum foil, and give half-strength MS for rice plants. Grow them in a glasshouse or growth chamber (28 °C light for 12-h/20 °C dark for 12-h).
Preparation of Striga seeds (preconditioning). At 1–2 weeks before infection day, surface-sterilize Striga seeds by immersing them in a 20% commercial bleach solution diluted to contain approximately 1.2% sodium hypochlorite (NaOCl) for 5 min. Replace the solution at least once, wait for 5 min, and wash with sterile water more than five times. Place the sterilized seeds on the glass microfiber filters GF/A paper with 8–10 ml water in a petri dish (90 mm diameter), cover it with aluminum foil, and keep it in a 25 °C plant growth chamber for 1–2 weeks.
Striga infection. Before Striga infection, add germination stimulants (final concentration of 10 nM strigol, or 1 µM GR24) onto the filter paper with preconditioned Striga. Return the petri dish at 25 °C and incubate for 2–24 h in the dark. Open the rhizotron and place the Striga seeds carefully along the rice roots using forceps (5–10 mm distance between seeds, Fig. 2). Put the rhizotron lid back and secure the rhizotron with two rubber bands. Cover the root parts with aluminum foil. Place it back to the glasshouse or plant growth chamber. In the case of two hours exposure to germination stimulants, Striga germination can be observed at one day, penetration at two days, and xylem bridge formation at 3–5 days after infection. In the case of 24 h germination stimulant treatment, Striga germination can be readily observed before rice infection, and penetration and xylem bridge formation can be observed at one day and 3–4 days after infection, respectively.
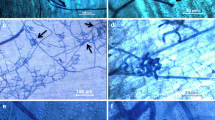
Quantification of Striga infection. After a few weeks of infection, Striga infection rates can be quantified depending on which stage you want to observe. The later stage can be analyzed at two and four weeks after infection. Carefully remove the rubber bands and the lid from a rhizotron without disturbing host roots. Observe the infection stage of each Striga seedling under a stereo microscope or magnification lenses. Count the number of Striga in each stage. Striga infection can be classified into the following seven groups, (a) not germinated, (b) germinated but not attached, (c) penetrated, but the seed coat is still covered, (d) penetrated but dead, (e) 1–2 pairs of open leaves, (f) 3–5 pairs of open leaves or (g) more than six pairs of open leaves (Yoshida and Shirasu 2009) (Fig. 3).
Classification of Striga hermontica infection on a sorghum host. a Not germinated, b germinated but not attached, c penetrated, but the seed coat is still covered, d penetrated but dead, e 1–2 pairs of open leaves, f 3–5 pairs of open leaves, and g more than six pairs of open leaves. Scale bars: 1000 µm
Safranin O staining for the observation of xylem bridges. Prepare a fixation solution by combining 75% ethanol, 25% acetic acid, and 0.1% Safranin O. Prepare a clearing solution by dissolving 1 g chloral hydrate (C2H3Cl3O2) in 1 mL glycerol and 2 mL water.
Excise 1–2 cm of Striga-infected root segments with scissors. Place root samples in the fixation solution and vacuum infiltrate for 15 min. Wash with sterile water three times. Add enough 0.1% Safranin O stain to cover the sample and heat at 90 °C for 5 min. Wash with sterile water three times. Soak in the clearing solution overnight. Observe the xylem bridge formation under microscopy. Xylem cells are stained in red with Safranin O (Fig. 4). The staining can also be observed under fluorescent microscopy with the filter setting for red fluorescent protein (RFP).
Making Technovit 7100-embedded thin sections. Prepare an FAA fixative solution by combining 1 mL formaldehyde, 0.5 mL glacial acetic acid, 5 mL ethanol, and 3.5 mL water. Prepare the following steps at room temperature. Cut the samples and put them into the FAA fixative solution, vacuum infiltrate for 15 min and leave at room temperature for more than two hours (place at 4 °C if you do it overnight). Dehydrate using an ethanol series. Replace fixative solution with 70% ethanol and incubate for 30 min with gentle agitation at room temperature. Next, replace 70% ethanol with 100% ethanol and incubate for 30 min with gentle agitation at room temperature. Repeat 100% ethanol incubation one more time.
Add 1/4 volume (250 µL for 1 mL ethanol) of Technovit 7100 liquid (Heraeus Kulzer GmbH; liquid, Hardener I, Hardener II), and incubate for 30 min with gentle agitation. Repeat until the solution contains an equal volume of Technovit 7100 liquid and ethanol (four times). Incubate for more than one hour (or overnight) with gentle agitation. Remove half the volume of Technovit 7100 liquid: ethanol solution. Add the same volume of Technovit 7100 liquid as you removed. Incubate for 30 min with gentle agitation. Prepare Technovit infiltration solution (add 1 g of Hardener I to 100 mL Technovit 7100 liquid). Replace the solution containing the sample with the Technovit infiltration solution. Incubate it for more than one hour with gentle agitation. Replace the solution with a new Technovit infiltration solution. Incubate it overnight with gentle agitation. Replace the solution with a new Technovit infiltration solution. Incubate it for 1–2 h(s) with gentle agitation. Prepare the Technovit embedding solution by mixing 1 mL Technovit infiltration solution and 66 µL Hardener II (this will start solidifying soon).
To embed the infiltrated Striga/root sample, cut it under a microscope to the appropriate size and shape. Prepare a proper mold (e.g., cap of 1.5 ml Eppendorf tube, etc.). Put the sample into the mold and add the Technovit embedding solution. Adjust the angle of samples. Leave it at room temperature (RT) until the sample becomes solid (1 h at RT or 37 °C, or overnight at 4 °C or RT).
Prepare a Technovit 3040 (Heraeus Kulzer GmbH; powder, liquid) mounting solution by mixing well Technovit 3040 powder and liquid at a 2:1 ratio under a fume hood. Pour the Technovit 3040 solution onto the embedded sample's surface and attach a small wood block to the solution (Optional: a quick-drying glue can be used). Leave the samples until the Technovit 3040 solution becomes solid.
For preparing thin sections of the sample for microscopic observation, preheat the slide warmer to 42 °C. Place water droplets onto a MAS-coated slide glasses to the approximate size of the sections. Insert the wood block with sample into the sample holder of a Manual Rotary Microtome (Leica RM2235 or equivalent) and a TC-65 Tungsten blade to the blade holder of the microtome. Carefully slice the sample with manual rotation to 2–4 µm thickness. Hold the section with thin forceps and place each section onto the water droplet on the slide glass. Check the section under a light microscope. Place the slide glass onto the preheated slide warmer until the water dries up.
Double staining with Safranin-O and Fast Green. Preheat a hot plate (Corning 6795-420D hot plate stir or equivalent) to about 80 °C. Drop 0.1% Safranin solution on the slide containing the sample sections to cover them. Place the slide on the heat block until the Safranin solution is almost dried up. Briefly wash the slide glass with water. Set the slide(s) in a staining basket. Prepare the glass jars each filled with water, 0.5% picric acid, 95% ethanol, 95% ethanol with few drops of HCl, 100% ethanol, 0.1% Fast Green FCF (Fujifilm Wako Pure Chemical Industries, Ltd.) and 100% ethanol. Wash with water until no more red pigment comes off from the slide. Dip the slide glass in 0.5% picric acid in 95% ethanol for a few seconds. Next, dip the slide glass in 95% EtOH with ammonium solution for a few seconds. Dip the slide glass in 95% EtOH with a few drops of HCl. Wash with 100% ethanol. Stain with 0.1% Fast Green FCF for 30 s to 1 min (ideal duration may vary). Wash with 100% ethanol. Place the slide glasses on the slide glass holder and dry them in the air, and observe with a bright field microscope (Fig. 5a).
Thin-sections Striga hermonthica infecting a rice root stained by different methods: a Safranin O and Fast Green double-stained cross-section of Striga hermonthica infecting a rice root. Scale bar, 50 µm. b toluidine blue-stained longitudinal section of Striga hermonthica infecting a rice root. S: Striga, H: rice
Simple staining with toluidine blue. The section on the slide glass can be stained with toluidine blue solution (Fig. 5b) instead of the double stainng listed above (Fig. 5a). Drop 0.01% toluidine blue solution (100 mg Toluidine Blue [Sigma-Aldrich] in 10 mL water) on the top of slide glass to cover the specimen. Wait for 2 min. Wash the slide glass by immersing in water. Observe with a bright field microscope.
Conclusion
The protocols herein described are useful for characterizing post-attachment Striga resistance in rice and sorghum. They may also be applied to maize with some modification (e.g., using larger plates to construct the rhizotrons). Although they can indicate pre-attachment resistance mechanisms, for instance by applying ungerminated preconditioned Striga seeds to host roots in the rhizotron, such observations are perhaps more suitable in the agar system described in Chapter “An Agar-Based Method for Determining Mechanisms of Striga Resistance in Sorghum”. The application of both co-culture methods can pinpoint the point at which resistance gained through mutagenesis counters Striga parasitism in cereal mutant lineages.
References
Gurney AL, Slate J, Press MC, Scholes JD (2006) A novel form of resistance in rice to the angiosperm parasite Striga hermonthica. New Phytol 169:199–208
Yoshida S, Shirasu K (2009) Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol 183:180–189
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the IAEA: International Atomic Energy Agency, its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the IAEA: International Atomic Energy Agency, provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the IAEA: International Atomic Energy Agency that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the IAEA: International Atomic Energy Agency's name for any purpose other than for attribution, and the use of the IAEA: International Atomic Energy Agency's logo, shall be subject to a separate written license agreement between the IAEA: International Atomic Energy Agency and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 IAEA: International Atomic Energy Agency
About this chapter
Cite this chapter
Muttaqin, M., Cui, S., Yoshida, S. (2024). Histological Analysis of Striga Infected Plants. In: Ghanim, A.M.A., Sivasankar, S., Rich, P.J. (eds) Mutation Breeding and Efficiency Enhancing Technologies for Resistance to Striga in Cereals. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-68181-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-68181-7_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-68180-0
Online ISBN: 978-3-662-68181-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)