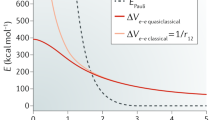
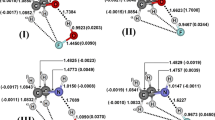
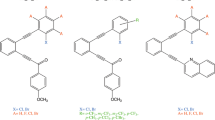
Abstract
This chapter deals with the application of valence bond theory in heterocyclic chemistry. A short introduction to the different valence bond methods is given, followed by illustrative results obtained by valence bond calculations. The illustrations show the applicability of the valence bond theory to obtain detailed information on the electronic structure of molecules in terms of chemical concepts.

Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Coulson CA (1961) Valence. Oxford University Press, London
Hall GG (1991) The Lennard-Jones paper of 1929 and the foundations of molecular orbital theory. In: Löwdin P-O, Sabin JR, Zerner MC (eds) Advances in quantum chemistry, vol 22. Academic Press, San Diego, pp 1–6
Hund F (1927) Zur Deutung der Molekelspektren. I. Z Physik 40:742–764
Lennard-Jones JE (1929) The electronic structure of some diatomic molecules. Trans Faraday Soc 25:668–686
Mulliken RS (1928) The assignment of quantum numbers for electrons in molecules. I. Phys Rev 32:186–222
Boys SF (1960) Construction of some molecular orbitals to be approximately invariant for changes from one molecule to another. Rev Mod Phys 32:296–299
Edmiston C, Ruedenberg K (1965) Localized atomic and molecular orbitals. II. J Chem Phys 43:S97–S116
Pipek J, Mezey PG (1989) A fast intrinsic localization procedure applicable for ab initio and semiempirical linear combination of atomic orbital wave functions. J Chem Phys 90:4916–4926
von Niessen W (1972) Density localization of atomic and molecular orbitals. I. J Chem Phys 56:4290–4297
Hiberty PC, Shaik S (2007) A survey of recent developments in ab initio valence bond theory. J Comput Chem 28:137–151
Shaik S, Hiberty PC (2004) Valence Bond, its history, fundamentals, and applications: a primer. In: Lipkowitz KB, Larter R, Cundary TR (eds) Reviews in computational chemistry. Wiley-VCH, New York, pp 1–100
Lewis GN (1916) The atom and the molecule. J Am Chem Soc 38:762–785
Heitler W, London F (1927) Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik. Z Physik 44:455–472
Heitler W, Rumer G (1931) Quantentheorie der chemischen Bindung für mehratomige Moleküle. Z Physik 68:12–41
Slater JC (1931) Directed valence in polyatomic molecules. Phys Rev 37:481–489
Pauling L (1931) The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J Am Chem Soc 53:1367–1400
Pauling L (1960) The nature of the chemical bond. Cornell University Press, Ithaca
Gallup GA (2002) Valence bond methods: theory and applications. Cambridge University Press, Cambridge
Chirgwin BH, Coulson CA (1950) The electronic structure of conjugated systems. 6. Proc R Soc Lond Ser A 201:196–209
Gallup GA, Norbeck JM (1973) Population analyses of valence-bond wavefunctions and BeH2. Chem Phys Lett 21:495–500
Pauling L, Wheland GW (1933) The nature of the chemical bond. V. The quantum-mechanical calculation of the resonance energy of benzene and naphthalene and the hydrocarbon free radicals. J Chem Phys 1:362–374
Broer R, Hozoi L, Nieuwpoort WC (2003) Non-orthogonal approaches to the study of magnetic interactions. Mol Phys 101:233–240
Hozoi L, de Vries AH, Broer R, de Graaf C, Bagus PS (2006) Ni 3s-hole states in NiO by non-orthogonal configuration interaction. Chem Phys 331:178–185
Coulson CA, Fischer I (1949) XXXIV. Notes on the molecular orbital treatment of the hydrogen molecule. Philos Mag 40:386–393
Goddard WA, Dunning TH, Hunt WJ, Hay PJ (1973) Generalized valence bond description of bonding in low-lying states of molecules. Acc Chem Res 6:368–376
Hay PJ, Hunt WJ, Goddard WA (1972) Generalized valence bond description of simple alkanes, ethylene, and acetylene. J Am Chem Soc 94:8293–8301
Hunt WJ, Hay PJ, Goddard WA (1972) Self-consistent procedures for generalized valence bond wavefunctions. Applications H3, BH, H2O, C2H6, and O2. J Chem Phys 57:738–748
Moss BJ, Bobrowicz FW, Goddard WA (1975) The generalized valence bond description of O2. J Chem Phys 63:4632–4639
Cooper DL, Gerratt J, Raimondi M, Wright SC (1987) The electronic structure of 1,3-dipoles: spin-coupled descriptions of nitrone and diazomethane. Chem Phys Lett 138:296–302
Cooper DL, Gerratt J, Raimondi M (1984) Studies of molecular states using spin-coupled valence-bond theory, Faraday Symp. Chem Soc 19:149–163
Cooper DL, Gerratt J, Raimondi M (1987) Modern valence bond theory. In: Lawley KP (ed) Advances in chemical physics: Ab initio methods in quantum chemistry, vol 69. Wiley, London, pp 319–397
Gerratt J (1971) General theory of spin-coupled wavefunctions for atoms and molecules. In: Bates D, Esterman I (eds) Advances in atomic and molecular physics, vol 7. Academic, New York, pp 141–221
Gerratt J, Raimondi M (1980) The spin-coupled valence bond theory of molecular electronic structure. I. Basic theory and application to the 2Σ+ states of BeH. Proc R Soc Lond A 371:525–552
Small DW, Head-Gordon M (2011) Post-modern valence bond theory for strongly correlated electron pairs. Phys Chem Chem Phys 13:19285–19297
Small DW, Head-Gordon M (2009) Tractable spin-pure methods for bondbreaking: local many-electron spin-vector sets and an approximate valence bond model. J Chem Phys 130:084103
van Lenthe JH, Balint-Kurti GG (1980) The valence-bond SCF (VB SCF) method: synopsis of theory and test calculation of OH potential energy curve. Chem Phys Lett 76:138–142
van Lenthe JH, Balint-Kurti GG (1983) The valence-bond self-consistent field method (VB-SCF): theory and test calculations. J Chem Phys 78:5699–5713
van Lenthe JH, Dijkstra F, Havenith RWA (2002) TURTLE – a gradient VBSCF program. Theory and studies of aromaticity. In: Cooper DL (ed) Valence bond theory, vol 10, Theoretical and computational chemistry. Elsevier, Amsterdam, pp 79–112
Dijkstra F, van Lenthe JH (1999) Gradients in valence bond theory. Chem Phys Lett 310:553–556
Dijkstra F, van Lenthe JH (2000) Gradients in valence bond theory. J Chem Phys 113:2100–2108
Havenith RWA (2005) Coupled valence bond theory. Chem Phys Lett 414:1–5
Zielinski ML, van Lenthe JH (2010) Atoms in valence bond – AiVB. Synopsis and test results. Chem Phys Lett 500:155–160
Rashid Z, van Lenthe JH, Havenith RWA (2012) Resonance and aromaticity: an ab initio valence bond approach. J Phys Chem A 116:4778–4788
Hiberty PC (1997) Reconciling simplicity and accuracy: compact valence bond wave functions with breathing orbitals. J Mol Struct (Theochem) 398–399:35–43
Hiberty PC, Flament JP, Noizet E (1992) Compact and accurate valence bond functions with different orbitals for different configurations: application to the two-configuration description of F2. Chem Phys Lett 189:259–265
Hiberty PC, Humbel S, Byrman CP, van Lenthe JH (1994) Compact valence bond functions with breathing orbitals: application to the bond dissociation energies of F2 and FH. J Chem Phys 101:5969–5976
Hiberty PC, Shaik S (2002) Breathing-orbital valence bond method – a modern valence bond method that includes dynamic correlation. Theor Chem Acc 108:255–272
Mo Y (2009) The resonance energy of benzene: a revisit. J Phys Chem A 113:5163–5169
Mo Y, Peyerimhoff SD (1998) Theoretical analysis of electronic delocalization. J Chem Phys 109:1687–1697
Mo Y, von R. Schleyer P (2006) An energetic measure of aromaticity and antiaromaticity based on the Pauling-Wheland resonance energies. Chem Eur J 12:2009–2020
Zielinski M, Havenith RWA, Jenneskens LW, van Lenthe JH (2010) A comparison of approaches to estimate the resonance energy. Theor Chem Acc 127:19–25
Roos BO (1987) The complete active space self-consistent field method and its applications in electronic structure calculations. Adv Chem Phys 69:399–445
Angeli C, Cimiraglia R, Malrieu J-P (2008) On the relative merits of nonorthogonal and orthogonal valence bond methods illustrated on the hydrogen molecule. J Chem Educ 85:150–158
Malrieu J-P, Guihéry N, Calzado CJ, Angeli C (2007) Bond electron pair: its relevance and analysis from the quantum chemistry point of view. J Comput Chem 28:35–50
Löwdin P-O (1950) On the non-orthogonality problem connected with the use of atomic wave functions in the theory of molecules and crystals. J Chem Phys 18:365–375
Löwdin P-O (1967) Group algebra, convolution algebra, and applications to quantum mechanics. Rev Mod Phys 39:259–287
Benker D, Klapötke TM, Kuhn G, Li J, Miller C (2005) An ab initio valence bond (VB) calculation of the π delocalisation energy in borazine, B3N3H6. Heteroat Chem 16:311–315
Engelberts JJ, Havenith RWA, van Lenthe JH, Jenneskens LW, Fowler PW (2005) The electronic structure of inorganic benzenes: valence bond and ringcurrent descriptions. Inorg Chem 44:5266–5272
Soncini A, Domene C, Engelberts JJ, Fowler PW, Rassat A, van Lenthe JH, Havenith RWA, Jenneskens LW (2005) A unified orbital model of delocalised and localised currents in monocycles, from annulenes to azabora-heterocycles. Chem Eur J 11:1257–1266
Cooper DL, Wright SC, Gerratt J, Hyams PA, Raimondi M (1989) The electronic structure of heteroaromatic molecules. Part 3. A comparison of benzene, borazine and boroxine. J Chem Soc Perkin Trans 2:719–724
Cyrañski M, von R. Schleyer P, Krygowski TM, Jiao H, Hohlneicher G (2003) Facts and artifacts about aromatic stability estimation. Tetrahedron 59:1657–1665
Steiner E, Fowler PW, Havenith RWA (2002) Current densities of localized and delocalized electrons in molecules. J Phys Chem A 106:7048–7056
Karadakov PB, Ellis M, Gerratt J, Cooper DL, Raimondi M (1997) The electronic structure of borabenzene: combination of an aromatic π-sextet and a reactive σ-framework. Int J Quantum Chem 63:441–449
Cooper DL, Wright SC, Gerratt J, Raimondi M (1989) The electronic structure of heteroaromatic molecules. Part 1. Six-membered rings. J Chem Soc Perkin Trans 2:255–261
Cooper DL, Gerratt J, Raimondi M (1986) The electronic structure of the benzene molecule. Nature 323:699–701
Wu JI, Wannere CS, Mo Y, von R. Schleyer P, Bunz UHF (2009) 4n π electrons but stable: N, N,-dihydrodiazapentacenes. J Org Chem 74:4343–4349
Cooper DL, Wright SC, Gerratt J, Raimondi M (1989) The electronic structure of heteroaromatic molecules. Part 2. Five-membered rings. J Chem Soc Perkin Trans 2:263–267
von R. Schleyer P, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJR (1996) Nucleus-Independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Karadakov PB, Gerratt J, Cooper DL, Raimondi M (1995) Modern valence bond description of bonding in strained three-membered rings: cyclopropane, aziridine, ethene oxide, phosphirane and thiirane. J Mol Struct (Theochem) 341:13–24
Walsh AD (1949) The structures of ethylene oxide, cyclopropane, and related molecules. Trans Faraday Soc 45:179–190
Cremer D, Gauss J (1986) Theoretical determination of molecular structure and conformation. 20. Reevaluation of the strain energies of cyclopropane and cyclobutane carbon-carbon and carbon-hydrogen bond energies, 1,3 interactions, and σ-aromaticity. J Am Chem Soc 108:7467–7477
Coulson CA, Moffitt WE (1949) I. The properties of certain strained hydrocarbons. Philos Mag 40:1–35
Braïda B, Lo A, Hiberty PC (2012) Can aromaticity coexist with diradical character? An ab initio valence bond study of S2N2 and related 6π-electron four membered rings E2N2 and E4 2+ (E= S,Se,Te). Chem Phys Chem 13:811–819
Gerratt J, McNicholas SJ, Karadakov PB, Sironi M, Raimondi M, Cooper DL (1996) The extraordinary electronic structure of N2S2. J Am Chem Soc 118:6472–6476
Thorsteinsson T, Cooper DL (1998) Nonorthogonal weights of modern VB wavefunctions. Implementation and applications within CASVB. J Math Chem 23:105–126
Scheschkewitz D, Amii H, Gornitzka H, Schoeller WW, Bourissou D, Bertrand G (2002) Singlet diradicals: from transition states to crystalline compounds. Science 295:1880–1881
Jung Y, Head-Gordon M (2003) How diradicaloid is a stable diradical? Chem Phys Chem 4:522–525
Jung Y, Head-Gordon M (2003) Controlling the extent of diradical character by utilizing neighboring group interactions. J Phys Chem A 107:7475–7481
Seierstad M, Kinsinger CR, Cramer CJ (2002) Design of 1,3-diphospha-2,4-diboretane diradicals. Angew Chem Int Ed 41:3894–3896
Havenith RWA, van Lenthe JH, van Walree CA, Jenneskens LW (2006) Orbital interactions expressed in resonance structures: an approach to compute stabilisation of cyclobutanediyl diradicals. J Mol Struct (Theochem) 763:43–50
Angeli C, Calzado CJ, de Graaf C, Caballol R (2011) The electronic structure of Ullman's biradicals: an orthogonal valence bond interpretation. Phys Chem Chem Phys 13:14617–14628
Mo Y (2010) Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect. Nat Chem 2:666–671
Wu W, Song L, Cao Z, Zhang Q, Shaik S (2002) Valence bond configuration interaction: a practical ab initio valence bond method that incorporates dynamic correlation. J Phys Chem A 106:2721–2726
Chen Z, Song J, Shaik S, Hiberty PC, Wu W (2009) Valence bond perturbation theory. A valence bond method that incorporates perturbation theory. J Phys Chem A 113:11560–11569
Song J, Wu W, Zhang Q, Shaik S (2004) A practical valence bond method: a configuration interaction method approach with perturbation theoretic facility. J Comput Chem 25:472–478
Rashid Z, van Lenthe JH (2013) A quadratically convergent VBSCF method. J Chem Phys 138:54105
Acknowledgement
RWAH and RB acknowledge the Zernike Institute for Advanced Materials for the financial support (“Dieptestrategie” programme).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rashid, Z., Broer, R., van Lenthe, J.H., Havenith, R.W.A. (2014). Valence Bond Theory in Heterocyclic Chemistry. In: De Proft, F., Geerlings, P. (eds) Structure, Bonding and Reactivity of Heterocyclic Compounds. Topics in Heterocyclic Chemistry, vol 38. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-45149-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-45149-2_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-45148-5
Online ISBN: 978-3-642-45149-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)