Abstract
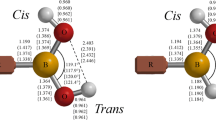
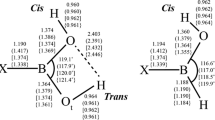
The chemistry of boric acid and monomeric borates is reviewed. Following a discussion of the crystal structures and nuclear magnetic resonance studies, ab initio results are presented of molecular ortho- and metaboric acid, (tetrahydroxo)borate, and the hydrates of orthoboric acid and borate. The structures and vibrational frequencies are compared with experiment. Attempts to study their interconversion lead us to a discussion of oxodihydroxoborate (the conjugate base of boric acid), and of the hydroxide-boric acid complex. It is hypothesized that the conversion of boric acid into borate proceeds via the oxodihydroxoborate intermediate. Finally, the calculated structures of hydroxodioxo- and trioxoborate are compared with experiment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Richens DT (1997) The chemistry of aqua ions. Wiley, Chichester
Oi T (2000) Calculations of reducted partition function ratios of monomeric and dimeric boric acids and borates by the ab initio molecular orbital theory. J Nucl Sci Tech 37(2):166–172
Oi T (2000) Ab initio molecular orbital calculations of reduced partition function ratios of polyboric acids and polyborate anions. Z Naturforsch A 55:623–628
Oi T, Yanase S (2001) Calculations of reduced partition function ratios of hydrated monoborate anion by the ab initio molecular orbital theory. J Nucl Sci Tech 38:429–432
Zeebe RE (2005) Stable boron isotope fractionation between dissolved B(OH)3 and B(OH)4−. Geochim Cosmochim Acta 69:2753–2766
Liu Y, Tossell JA (2005) Ab initio molecular orbital calculations for boron isotope fractionation on boric acids and borates. Geochim Cosmochim Acta 69:3995–4006
Tossell JA (2005) Boric acid, “carbonic” acid, and N-containing oxyacids in aqueous solution: ab initio studies of structure, pKa, NMR shifts, and isotopic fractionation. Geochim Cosmochim Acta 69:5647–5658
Rustad JR, Bylaska EJ (2007) Ab initio calculation of isotopic fractionation in B(OH)3(aq) and B(OH)4−(aq). J Am Chem Soc 129:2222–2223
Rustad JR, Bylaska EJ, Jackson VE, Dixon DA (2010) Calculation of boron-isotope fractionation between B(OH)3(aq) and B(OH)4-(aq). Geochim Cosmochim Acta 74:2843–2850
Zeebe RE, Sanyal A, Ortiz JD, Wolf-Gladrow DA (2001) A theoretical study of the kinetics of the boric acid-borate equilibrium in seawater. Marine Chem 73:113–124
Kracek FC, Morey GW, Merwin HE (1938) The system, water-boron oxide. Am J Sci A 35:143–171
Blasdale WC, Slansky CM (1939) The solubility curves of boric acid and the borates of sodium. J Am Chem Soc 61:917–920
Berger SV (1953) The crystal structure of boron oxide. Acta Chem Scand 7:611–622
Strong SL, Kaplow R (1968) The structure of crystalline B2O3. Acta Crystallogr B 24:1032–1036
Gurr GE, Montgomery PW, Knutson CD, Gorres BT (1970) The crystal structure of trigonal diboron trioxide. Acta Crystallogr B 26:906–915
Effenberger H, Lengauer CL, Parthe E (2001) Trigonal B2O3 with higher space-group symmetry: results of a reevaluation. Monat Chem 132:1515–1517
Prewitt CT, Shannon RD (1968) Crystal structure of a high-pressure form of B2O3. Acta Crystallogr B 24:869–874
Zachariasen WH (1934) The crystal lattice of boric acid, BO3H3. Z Kristallogr 88:150–161
Cowley JM (1953) Structure analysis of single crystals by electron diffraction. II. Disordered boric acid structure. Acta Crystallogr 6:522–529
Zachariasen WH (1954) The precise structure of orthoboric acid. Acta Crystallogr 7:305–310
Shuvalov RR, Burns PC (2003) A new polytype of orthoboric acid, H3BO3-3T1. Acta Crystallogr C 59:i47–i49
Tazaki H (1940) Single crystals of metaboric acid. J Sci Hiroshima Univ A 10:37–54
Tazaki H (1940) The structure of orthorhombic metaboric acid, HBO2(a). J Sci Hiroshima Univ A 10:55–61
Peters CR, Milberg ME (1964) The refined structure of orthorhombic metaboric acid. Acta Crystallogr 17:229–234
Zachariasen WH (1952) A new analytical method for solving complex crystal structures. Acta Crystallogr 5:68–73
Zachariasen WH (1963) The crystal structure of monoclinic metaboric acid. Acta Crystallogr 16:385–389
Freyhardt CC, Wiebcke M, Felsche J (2000) The monoclinic and cubic phases of metaboric acid (precise redeterminations). Acta Crystallogr C 56:276–278
Zachariasen WH (1963) The crystal structure of cubic metaboric acid. Acta Crystallogr 16:380–384
Konig H, Hoppe R (1977) Zur Kenntnis von Na3BO3. Z Anorg Allg Chem 434:225–232
Menchetti S, Sabelli C (1982) Structure of hydrated sodium borate Na2[BO2(OH)]. Acta Crystallogr B 38:1282–1284
Block S, Perloff A (1963) The direct determination of the crystal structure of NaB(OH)42H2O. Acta Crystallogr 16:1233–1238
Csetenyi LJ, Glasser FP, Howie RA (1993) Structure of sodium tetrahydroxyborate. Acta Crystallogr C 49:1039–1041
Touboul M, Betourne E, Nowogrocki G (1995) Crystal structure and dehydration process of Li(H2O)4B(OH)4.2H2O. J Solid State Chem 115:549–553
Zachariasen WH (1964) The crystal structure of lithium metaborate. Acta Crystallogr 17:749–751
Hohne E (1964) Die Kristallstruktur des LiB(OH)4. Z Chem 4:431–432
Fronczek FR, Aubry DA, Stanley GG (2001) Refinement of lithium tetrahydroxoborate with low-temperature CCD data. Acta Crystallogr E 57:i62–i63
Onak TP, Landesman H, Williams RE, Shapiro I (1959) The B11 nuclear magnetic resonance chemical shifts and spin coupling values for various compounds. J Phys Chem 63:1533–1535
Momii RK, Nachtrieb NH (1967) Nuclear magnetic resonance study of borate-polyborate equilibria in aqueous solution. Inorg Chem 6:1189–1192
How MJ, Kennedy GR, Mooney EF (1969) The pH dependence of the boron-11 chemical-shift of borate-boric acid solutions. J Chem Soc D Chem Commun 267–268
Smith HDJ, Wiersema RJ (1972) Boron-11 nuclear magnetic resonance study of polyborate ions in solution. Inorg Chem 11:1152–1154
Covington AK, Newman KE (1973) Base dissociation constant of the borate ion from 11B chemical shifts. J Inorg Nucl Chem 35:3257–3262
Henderson WG, How MJ, Kennedy GR, Mooney EF (1973) The interconversion of aqueous boron species and the interaction of borate with diols: a 11B N.M.R. study. Carbohydrate Res 28:1–12
Janda R, Heller G (1979) 11B–NMR-spektroskopische Untersuchungen an waessrigen Polyboratloesungen. Z Naturforsch B 34:1078–1083
Epperlein BW, Lutz O, Schwenk A (1975) Fourier-Kernresonanzuntersuchungen an 10B und 11B in Waessriger Loesung. Z Naturforsch A 30:955–958
Salentine CG (1983) High-field 11B NMR of alkali borates. Aqueous polyborate equilibria. Inorg Chem 22:3920–3924
Frisch MJ et al (2004) Gaussian 03, Revision D.02. Gaussian Inc., Wallingford, CT
Gupta A, Tossell JA (1981) A theoretical study of bond distances, X-ray spectra and electron density distributions in borate polyhedra. Phys Chem Miner 7:159–164
Gupta A, Tossell JA (1983) Quantum mechanical studies of distortions and polymerization of borate polyhedra. Am Miner 68:989–995
Zhang ZG, Boisen MBJ, Finger LW, Gibbs GV (1985) Molecular mimicry of the geometry and charge density distribution of polyanions in borate minerals. Am Miner 70:1238–1247
Zaki K, Pouchan C (1995) Vibrational analysis of orthoboric acid H3BO3 from ab initio second-order perturbation calculations. Chem Phys Lett 236:184–188
Tian SX, Xu KZ, Huang M-B, Chen XJ, Yang JL, Jia CC. Theoretical study on infrared vibrational spectra of boric-acid in gas-phase using density functional methods. J Mol Struct (Theochem) 459:223–227, 459
Tachikawa M (2004) A density functional study on hydrated clusters of orthoboric acid, B(OH)3(H2O)n (n = 1–5). J Mol Struct (Theochem) 710:139–150
Stefani D, Pashalidis I, Nicolaides AV (2008) A computational study of the conformations of the boric acid (B(OH)3), its conjugate base ((HO)2BO–) and borate anion (B(OH)4–). J Mol Struct (Theochem) 853:33–38
Zhou Y, Fang C, Fang Y, Zhu F (2011) Polyborates in aqueous borate solution: a Raman and DFT theory investigation. Spectrochim Acta A 83:82–87
Ananthakrishnan R (1936) The Raman spectra of some boron compounds (methyl borate, ethyl borate, boron tri-bromide and boric acid). Proc Indian Acad Sci A 4:74–81
Ananthakrishnan R (1937) The Raman spectra of crystal powders. IV. Some organic and inorganic compounds. Proc Indian Acad Sci A 5:200–221
Hibben JH (1938) The constitution of some boric oxide compounds. Am J Sci A 35:113–125
Mitra SM (1938) Raman effect in boric acid and in some boron compounds. Ind J Phys 12:9–14
Kahovec L (1938) Studien zum Raman-Effekt. Mitteilung LXXXV. Borsauere und Derivate. Z Phys Chem 40:135–145
Miller FA, Wilkins CH (1952) Infrared spectra and characteristic frequencies of inorganic ions. Anal Chem 24:1253–1294
Bethell DE, Sheppard N (1955) The infra-red spectrum and structure of boric acid. Trans Faraday Soc 51:9–15
Servoss RR, Clark HM (1957) Vibrational spectra of normal and isotopically labeled boric acid. J Chem Phys 26:1175–1178
Maya L (1976) Identification of polyborate and fluoropolyborate ions in solution by Raman spectroscopy. Inorg Chem 15:2179–2184
Maeda M, Hirao T, Kotaka M, Kakihana H (1979) Raman spectra of polyborate ions in aqueous solution. J Inorg Nucl Chem 41:1217–1220
Janda R, Heller G (1979) Ramanspektroskopische Untersuchungen an festen und in Wasser geloesten Polyboraten. Z Naturforsch B 34:585–590
Ogden JS, Young NA (1988) The characterisation of molecular boric acid by mass spectrometry and matrix isolation infrared spectroscopy. J Chem Soc Dalton Trans 1645–1652
Gilson TR (1991) Characterization of ortho- and meta-boric acids in the vapour phase. J Chem Soc Dalton Trans 2463–2466
Andrews L, Burkholder TR (1992) Infrared spectra of molecular B(OH)3 and HOBO in solid argon. J Chem Phys 97:7203–7210
Gupta A, Swanson DK, Tossell JA, Gibbs GV (1981) Calculation of bond distances, one-electron properties and electron density distributions in first-row tetrahedral hydroxy and oxyanions. Am Miner 66:601–609
Hess AC, McMillan PF, O’Keeffe M (1988) Torsional barriers and force fields in H4TO4 molecules and molecular ions (T = C, B, Al, Si). J Phys Chem 92:1785–1791
Nielsen JR, Ward NE (1937) Raman spectrum and structure of the metaborate ion. J Chem Phys 5:201
Edwards JO, Morrison GC, Ross VF, Schultz JW (1955) The structure of the aqueous borate ion. J Am Chem Soc 77:266–268
Oertel RP (1972) Raman study of aqueous monoborate-polyol complexes. Equilibria in the monoborate-1,2-ethanediol system. Inorg Chem 11:544–549
Liu Z, Gao B, Hu M, Li S, **a S (2003) FT-IR and Raman spectroscopic analysis of hydrated cesium borates and their saturated aqueous solution. Spectrochim Acta A 59:2741–2745
Zhu FY, Fang CH, Fang Y, Zhou YQ, Ge HW, Liu HY (2014) Structure of aqueous potassium metaborate solution. J Mol Struct 1070:80–85
Attina M, Cacace F, Occhiucci G, Ricci A (1992) Gaseous borate and polyborate anions. Inorg Chem 31:3114–3117
Waton G, Mallo P, Candau SJ (1984) Temperature-jump rate study of the chemical relaxation of aqueous boric acid solutions. J Phys Chem 88:3301–3305
Acknowledgements
The author acknowledges the Atlantic Computational Excellence Network (ACEnet) for computational support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Pye, C.C. (2018). An Ab Initio Study of Boric Acid, Borate, and their Interconversion. In: Wang, Y., Thachuk, M., Krems, R., Maruani, J. (eds) Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics. Progress in Theoretical Chemistry and Physics, vol 31. Springer, Cham. https://doi.org/10.1007/978-3-319-74582-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-74582-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74581-7
Online ISBN: 978-3-319-74582-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)