Abstract
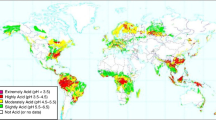
Achieving sustainable food production to feed the increasing population of the problematic lands of the world is an enormous challenge. Aluminum (Al) toxicity in the acid soil is a major worldwide problem. Liming and nutrient management technologies are worthless due to high lime requirement, and the effect of liming does not persist for long. Besides this, conventional breeding is useful to manage Al toxicity as some plants have evolved mechanisms to cope with Al toxicity in acid soil. Therefore, understanding of Al tolerance mechanisms is prime necessity for improving Al tolerance in crops. Al resistance mechanisms include mainly Al avoidance (Al exclusion) and/or Al tolerance (detoxification of Al inside the cell) mechanisms. In this chapter, we summarize Al behavior in plant root cell. We include recent findings of Al resistance mechanisms and Al-resistant genes which can be useful to produce cultivars adapted to acid soils.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Achary VMM, Panda BB (2010) Aluminium-induced DNA damage and adaptive response to genotoxic stress in plant cells are mediated through reactive oxygen intermediates. Mutagenesis 25:201–209
Achary VMM, Jena S, Panda KK, Panda BB (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70:300–310
Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H (2001) Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant Physiol 126:1381–1390
Akeson MA, Munns DN (1989) Lipid bilayer permeation by neutral aluminum citrate and by three α-hydroxy carboxylic acids. Biochim Biophys Acta 984:200–206
Amenós M, Corrales I, Poschenrieder C, Illéš P, Baluška F, Barcelo J (2009) Different effects of aluminium on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminium tolerance. Plant Cell Physiol 50:528–540
Basu U, Good AG, Taylor GJ (2001) Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ 24:1269–1278
Bennet RJ, Breen CM (1991) The aluminium signal: new dimensions to mechanisms of aluminium tolerance. Plant Soil 134:153–166
Blamey FPC, Asher CJ, Kerven GL, Edwards DG (1993) Factors affecting aluminium sorption by calcium pectate. Plant Soil 149:87–94
Blancaflor EB, Jones DL, Gilroy S (1998) Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol 118:159–172
Boscolo PRS, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22:1009–1017
Chen ZC, Yokosho K, Kashino M, Zhou F-J, Yamaji N, Ma JF (2013) Adaptation to acidic soil is achieved by increased numbers of cis-acting elements regulating ALMT1 expression in Holcus lanatus. Plant J 76(1):10–23
Clarkson DT (1965) The effect of aluminium and some other trivalent metal cations on cell division in the root apices of Allium cepa. Ann Bot 29:309–315
Collins NC, Shirley NJ, Saeed M, Pallotta M, Gustafson JP (2008) An ALMT1 gene cluster controlling aluminum tolerance at the Alt4 locus of rye (Secale cereale L.). Genetics 179:669–682
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Delhaize E, Ryan PR, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) (II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiol 103:695–702
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminium tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci U S A 101:15249–15254
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17:341–348
Dipierro N, Mondelli D, Paciolla C, Brunetti G, Dipierro S (2005) Changes in the ascorbate system in the response of pumpkin (Cucurbita pepo L.) roots to aluminium stress. J Plant Physiol 162:529–536
Doncheva S, Amenós M, Poschenrieder C, Barceló J (2005) Root cell patterning: a primary target for aluminium toxicity in maize. J Exp Bot 56:1213–1220
Exley C (2004) The pro-oxidant activity of aluminum. Free Radic Biol Med 36:380–387
Ezaki B, Yamamoto Y, Matsumoto H (1995) Cloning and sequencing of the cDNAs induced by aluminium treatment and Pi starvation in cultured tobacco cells. Physiol Plant 93:11–18
Ezaki B, TsugUa S, Matsumoto H (1996) Expression of a moderately anionic peroxidase is induced by aluminum treatment in tobacco cells: possible involvement of peroxidase isozymes in aluminum ion stress. Physiol Plant 96:21–28
Ezaki B, Katsuhara M, Kawamura M, Matsumoto H (2001) Different mechanisms of four aluminum (Al)-resistant transgenes for Al toxicity in Arabidopsis. Plant Physiol 127:918–927
Ezaki B, Sasaki K, Matsumoto H, Nakashima S (2005) Functions of two genes in aluminium (Al) stress resistance: repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1gene. J Exp Bot 56:2661–2671
Ezaki B, Nagao E, Yamamoto Y, Nakashima S, Enomoto T (2008) Wild plants, Andropogon virginicus L. and Miscanthus sinensis Anders, are tolerant to multiple stresses including aluminum, heavy metals and oxidative stresses. Plant Cell Rep 27:951–961
Fiskesjö G (1990) Occurrence and degeneration of ‘Al-structures’ in root cap cells of Allium cepa L. after Al-treatment. Hereditas 112:193–202
Food and Agricultural Organization (FAO) (2009) Global agriculture towards 2050. In: High level expert forum on How to feed the world in 2050, FAO, Rome
Foy CD (1988) Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Anal 19:959–987
Frantzios G, Galatis B, Apostolakos P (2000) Aluminium effects on microtubule organization in dividing root-tip cells of Triticum turgidum. I. Mitotic cells. New Phytol 145:211–224
Fujii M, Yokosho K, Yamaji N, Saisho D, Yamane M, Takahashi H, Sato K, Nakazono M, Ma JF (2012) Acquisition of aluminium tolerance by modification of a single gene in barley. Nat Commun 3:713
Furuichi T, Sasaki T, Tsuchiya Y, Ryan PR, Delhaize E, Yamamoto Y (2010) An extracellular hydrophilic carboxy-terminal domain regulates the activity of TaALMT1, the aluminum-activated malate transport protein of wheat. Plant J 64:47–55
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48:1081–1091
Gutteridge JMC, Quinlan GJ, Clark I, Halliwell B (1985) Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim Biophys Acta 835:441–447
Halliwell B, Gutteridge J (1989) Protection against oxidants in biological systems: the superoxide theory of oxygen toxicity. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Clarendon, Oxford, pp 86–187
He H, He L, Gu M (2012a) Interaction between nitric oxide and plant hormones in aluminium tolerance. Plant Signal Behav 7:469–471
He H, Zhan J, He L, Gu M (2012b) Nitric oxide signaling in aluminum stress in plants. Protoplasma 249:483–492
Hoekenga OA, Vision TJ, Shaff JE, Monforte AJ, Lee GP, Howell SH, Kochian LV (2003) Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta × Columbia) by quantitative trait locus map**. A physiologically simple but genetically complex trait. Plant Physiol 132:936–948
Hoekenga OA, Maron LG, Piñeros MA, Cançado GMA, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, Matsumoto H, Yamamoto Y, Koyama H, Kochian LV (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci 103:9738–9743
Horst WJ (1995) The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenkunde 158:419–428
Horst WJ, Püschel AK, Schmohl N (1997) Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant Soil 192:23–30
Horst W, Schmohl N, Kollmeier M, Baluska F, Sivaguru M (1999) Does aluminium affect root growth of maize through interaction with the cell wall—plasma membrane—cytoskeleton continuum? Plant Soil 215:163–174
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197
Hossain MA, Hossain AKMZ, Kihara T, Koyama H, Hara T (2005) Aluminum-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci Plant Nutr 51:223–230
Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF (2009) A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 21:655–667
Inostroza-Blancheteau C, Rengel Z, Alberdi M, Luz MM, Aquea F, Arce-Johnson P, Reyes-Díaz M (2012) Molecular and physiological strategies to increase aluminum resistance in plants. Mol Biol Rep 39:2069–2079
Ito D, Shinkai Y, Kato Y, Kondo T, Yoshida K (2009) Chemical studies on different color development in blue-and red-colored sepal cells of Hydrangea macrophylla. Biosci Biotechnol Biochem 73:1054–1059
Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci 104:9900–9905
Jones DL, Kochian LV (1995) Aluminum inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell Online 7:1913–1922
Jones DL, Kochian LV (1997) Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett 400:51–57
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29:1309–1318
Kinraide TB (1997) Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminium. J Exp Bot 48:1115–1124
Kinraide TB, Ryan PR, Kochian LV (1992) Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol 99:1461–1468
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Kochian L, Piñeros M, Hoekenga O (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Kollmeier M, Felle HH, Horst WJ (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122:945–956
Larsen P, Cancel J, Rounds M, Ochoa V (2007) Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 225:1447–1458
Li XF, Ma JF, Matsumoto H (2000) Pattern of aluminum induced secretion of organic acids differs between rye and wheat. Plant Physiol 123:1537–1544
Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H (2006) The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol 142:1294–1303
Ligaba A, Kochian L, Piñeros M (2009) Phosphorylation at S384 regulates the activity of the TaALMT1 malate transporter that underlies aluminum resistance in wheat. Plant J 60:411–423
Ligaba A, Dreyer I, Margaryan A, Schneider DJ, Kochian L, Piñeros M (2013) Functional, structural and phylogenetic analysis of domains underlying the Al sensitivity of the aluminium-activated malate/anion transporter, TaALMT1. Plant J 76:766–780
Lindberg S, Szynkier K, Greger M (1991) Aluminium effects on transmembrane potential in cells of fibrous roots of sugar beet. Physiol Plant 83:54–62
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57:389–399
Liu J, Piñeros MA, Kochian LV (2014) The role of aluminum sensing and signaling in plant aluminum resistance. J Integr Plant Biol 56:221–230
Llugany M, Poschenrieder C, Barceló J (1995) Monitoring of aluminium-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminium and proton toxicity. Physiol Plant 93:265–271
Ma Z, Miyasaka SC (1998) Oxalate exudation by taro in response to Al. Plant Physiol 118:861–865
Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H (1997a) Internal detoxification mechanism of Al in Hydrangea (identification of Al form in the leaves). Plant Physiol 113:1033–1039
Ma JF, Zheng SJ, Matsumoto H, Hiradate S (1997b) Detoxifying aluminum with buckwheat. Nature 390:569–570
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Ma Q, Rengel Z, Kuo J (2002) Aluminium toxicity in rye (Secale cereale): root growth and dynamics of cytoplasmic Ca2+ in intact root tips. Ann Bot 89:241–244
Ma B, Wan J, Shen Z (2007) H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regul 52:91–100
Ma JF, Chen ZC, Shen RF (2014) Molecular mechanisms of Al tolerance in gramineous plants. Plant Soil 381:1–12
Magalhaes JV (2002) Molecular genetic and physiological investigations of aluminum tolerance in sorghum (Sorghum bicolor L. Moench). Cornell University, Ithaca
Magalhaes JV, Liu J, Guimaraes CT, Lana UGP, Alves VMC, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39:1156–1161
Maron LG, Piñeros MA, Guimarães CT, Magalhaes JV, Pleiman JK, Mao C, Shaff J, Belicuas SNJ, Kochian LV (2010) Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J 61:728–740
Maron LG, Guimarães CT, Kirst M, Albert PS, Birchler JA, Bradbury PJ, Buckler ES, Coluccio AE, Danilova TV, Kudrna D, Magalhaes JV, Piñeros MA, Schatz MC, Wing RA, Kochian LV (2013) Aluminium tolerance in maize is associated with higher MATE 1 gene copy number. Proc Natl Acad Sci U S A 111:5241–5256
Massot N, Llugany M, Poschenrieder C, Barceló J (1999) Callose production as indicator of aluminum toxicity in bean cultivars. J Plant Nutr 22:1–10
Matsumoto H (1991) Biochemical mechanism of the toxicity of aluminium and the sequestration of aluminium in plant cells. In: Wright RJ, Baliga VC, Murrmann RP (eds) Plant-soil interactions at low pH. Springer Netherlands, Dordrecht, pp 825–838
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46, Academic
Matsumoto H, Motoda H (2012) Aluminum toxicity recovery processes in root apices. Possible association with oxidative stress. Plant Sci 185–186:1–8
Matsumoto H, Motoda H (2013) Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 363:399–410
Matsumoto H, Hirasawa E, Torikai H, Takahashi E (1976) Localization of absorbed aluminium in pea root and its binding to nucleic acids. Plant Cell Physiol 17:127–137
Matsumoto H, Morimura S, Takahashi E (1977a) Less involvement of pectin, in the precipitation of aluminium in pea root. Plant Cell Physiol 18:325–335
Matsumoto H, Morimura S, Takahashi E (1977b) Binding of aluminium to DNA of DNP in pea root nuclei. Plant Cell Physiol 18:987–993
Milla MAR, Butler E, Huete AR, Wilson CF, Anderson O, Gustafson JP (2002) Expressed sequence tag-based gene expression analysis under aluminum stress in rye. Plant Physiol 130:1706–1716
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Morgante M, De Paoli E, Radovic S (2007) Transposable elements and the plant pan-genomes. Curr Opin Plant Biol 10:149–155
Morimura S, Takahashi E, Matsumoto H (1978) Association of aluminium with nuclei and inhibition of cell division in onion (Allium cepa) roots. Z Pflanzenphysiol 88:395–401
Olivetti GP, Cumming JR, Etherton B (1995) Membrane potential depolarization of root cap cells precedes aluminum tolerance in snapbean. Plant Physiol 109:123–129
Ono K, Yamamoto Y, Hachiya A, Matsumoto H (1995) Synergistic inhibition of growth by aluminum and iron of tobacco (Nicotiana tabacum L.) cells in suspension culture. Plant Cell Physiol 36:115–125
Papernik LA, Kochian LV (1997) Possible involvement of Al-induced electrical signals in Al tolerance in wheat. Plant Physiol 115:657–667
Pellet D, Grunes D, Kochian L (1995) Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196:788–795
Pereira JF, Zhou G, Delhaize E, Richardson T, Zhou M, Ryan PR (2010) Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Ann Bot 106:205–214
Raman H, Zhang K, Cakir M, Appels R, Garvin DF, Maron LG, Kochian LV, Moroni JS, Raman R, Imtiaz M, Drake-Brockman F, Waters I, Martin P, Sasaki T, Yamamoto Y, Matsumoto H, Hebb DM, Delhaize E, Ryan PR (2005) Molecular characterization and map** of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 48:781–791
Rengel Z (1992) Disturbance of cell Ca2+ homeostasis as a primary trigger of Al toxicity syndrome. Plant Cell Environ 15:931–938
Rengel Z, Zhang WH (2003) Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol 159:295–314
Richards KD, Schott EJ, Sharma YK, Davis KR, Gardner RC (1998) Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol 116:409–418
Rincón M, Gonzales RA (1992) Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol 99:1021–1028
Roy AK, Sharma A, Talukder G (1989) A time-course study on effects of aluminium on mitotic cell division in Allium sativum. Mutat Res Lett 227:221–226
Ryan P, Delhaize E (2010) The convergent evolution of aluminium resistance in plants exploits a convenient currency. Funct Plant Biol 37:275–284
Ryan PR, Ditomaso JM, Kochian LV (1993) Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44:437–446
Ryan P, Delhaize E, Randall P (1995) Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Funct Plant Biol 22:531–536
Ryan P, Delhaize E, Jones D (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52:527–560
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351
Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang WH, Delhaize E (2011) The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J Exp Bot 62:9–20
Sasaki M, Yamamoto Y, Matsumoto H (1997) Aluminum inhibits growth and stability of cortical microtubules in wheat (Triticum aestivum) roots. Soil Sci Plant Nutr 43:469–472
Sasaki T, Ezaki B, Matsumoto H (2002) A gene encoding multidrug resistance (mdr)-like protein is induced by aluminum and inhibitors of calcium flux in wheat. Plant Cell Physiol 43:177–185
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Kawaura K, Noda K, Kojima T, Toyoda A, Matsumoto H, Yamamoto Y (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47:1343–1354
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell Environ 23:735–742
Schwarzerová K, Zelenková S, Nick P, Opatrný Z (2002) Aluminum-induced rapid changes in the microtubular cytoskeleton of tobacco cell lines. Plant Cell Physiol 43:207–216
Silva IR, Smyth TJ, Moxley DF, Carter TE, Allen NS, Rufty TW (2000) Aluminum accumulation at nuclei of cells in the root tip. Fluorescence detection using lumogallion and confocal laser scanning microscopy. Plant Physiol 123:543–552
Sivaguru M, Horst WJ (1998) The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol 116:155–163
Sivaguru M, Fujiwara T, Šamaj J, Baluška F, Yang Z, Osawa H, Maeda T, Mori T, Volkmann D, Matsumoto H (2000) Aluminum-induced 1 → 3-β-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol 124:991–1006
Sivaguru M, Pike S, Gassmann W, Baskin TI (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44:667–675
Sivaguru M, Liu J, Kochian LV (2013) Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminium resistance. Plant J 76(2):297–307
Stass A, Smit I, Eticha D, Oettler G, Johannes HW (2008) The significance of organic-anion exudation for the aluminum resistance of primary triticale derived from wheat and rye parents differing in aluminum resistance. J Plant Nutr Soil Sci 171:634–642
Tabuchi A, Matsumoto H (2001) Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112:353–358
Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K (2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307:167–178
Takeda K, Kariuda M, Itoi H (1985) Blueing of sepal colour of Hydrangea macrophylla. Phytochemistry 24:2251–2254
Tamás L, Huttová J, Mistrík I (2003) Inhibition of Al-induced root elongation and enhancement of Al-induced peroxidase activity in Al-sensitive and Al-resistant barley cultivars are positively correlated. Plant Soil 250:193–200
Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ (2000) Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol 123:987–996
Tolrà R, Vogel-Mikuš K, Hajiboland R, Kump P, Pongrac P, Kaulich B, Gianoncelli A, Babin V, Barceló J, Regvar M, Poschenrieder C (2011) Localization of aluminium in tea (Camellia sinensis) leaves using low energy X-ray fluorescence spectro-microscopy. J Plant Res 124:165–172
Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161:880–892
Verma DPS (2001) Cytokinesis and building of the cell plate in plants. Annu Rev Plant Physiol Plant Mol Biol 52:751–784
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Wagatsuma T, Akiba R (1989) Low surface negativity of root protoplasts from aluminum-tolerant plant species. Soil Sci Plant Nutr 35:443–452
Watt DA (2003) Aluminium‐responsive genes in sugarcane: identification and analysis of expression under oxidative stress. J Exp Bot 54:1163–1174
Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF (2009) A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell Online 21:3339–3349
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yamamoto Y, Kobayashi Y, Devi S, Rikiishi S, Matsumoto H (2003) Oxidative stress triggered by aluminum in plant roots. In: Abe J (ed) Roots: the dynamic interface between plants and the earth. Springer Netherlands, Dordrecht, pp 239–243
Yang ZM, Sivaguru M, Horst WJ, Matsumoto H (2000) Aluminium tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max). Physiol Plant 110:72–77
Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146:602–611
Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ (2011) Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol 155:1885–1892
Yin L, Mano J, Wang S, Tsuji W, Tanaka K (2010) The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol 152:1406–1417
Yokosho K, Yamaji N, Ma JF (2010) Isolation and characterisation of two MATE genes in rye. Funct Plant Biol 37:296–303
Yokosho K, Yamaji N, Ma JF (2011) An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J 68:1061–1069
Zhang Y (1995) Effects of aluminum chloride on the nucleus and nucleolus in root tip cells of Hordeum vulgare. Mutat Res 335:137–142
Zhang WH, Rengel Z (1999) Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Funct Plant Biol 26:401–409
Zhang J, He Z, Tian H, Zhu G, Peng X (2007) Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J Exp Bot 58:2269–2278
Zheng SJ (2010) Crop production on acidic soils: overcoming aluminium toxicity and phosphorus deficiency. Ann Bot 106:183–184
Zheng SJ, Yang JL (2005) Target sites of aluminum phytotoxicity. Biol Plant 49:321–331
Zhou G, Delhaize E, Zhou M, Ryan PR (2011) Biotechnological solutions for enhancing the aluminium resistance of crop plants. In: Shanker A, Venkateswarlu B (eds) Abiotic stress in plants—mechanisms and adaptations. InTech, Rijeka, pp 119–142
Zhou G, Delhaize E, Zhou M, Ryan PR (2013) The barley MATE gene, HvAACT, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann Bot 112:603–612
Acknowledgement
B.N.T. and B.E. thank the Department of Science and Technology, New Delhi, and JSPS, Japan, respectively, for financial support in the form of a project under Indo-Japan Science Cooperation Programme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Aggarwal, A., Ezaki, B., Munjal, A., Tripathi, B.N. (2015). Physiology and Biochemistry of Aluminum Toxicity and Tolerance in Crops. In: Tripathi, B., Müller, M. (eds) Stress Responses in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-13368-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-13368-3_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13367-6
Online ISBN: 978-3-319-13368-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)