Abstract
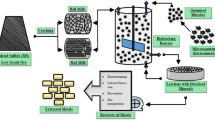
Extraction of metals (leaching) is chemical or biochemical processes that utilize acids or microorganisms to enhance the suspension of metals from the primary and secondary sources by making them more amenable to dissolution in aqueous solutions (leachate). Recovery of metals from the leachates is an essential stage supported by additional purification processes such as precipitation of impurities, electrowinning, solvent extraction, chemical or biological adsorption, and ion exchange. In this study, especially biosorption and metal sulfide precipitation are overviewed and discussed. Biosorption is a process by which particular biomass such as bacteria, fungi, yeast, agricultural wastes, algae, and biowastes can able to bind with specific ions or other molecules from aqueous solutions. Metal sulfide precipitation can be highly effective in obtaining a high degree of separation of metal cations from complex leachates. Each of these techniques has advantages and drawbacks. Sometimes, a technique may not be effective in attaining higher metal recovery. Therefore, different recovery techniques are needed to recover the target elements from the complex leachates. Maybe a combination of two or three recovery techniques is required to recover metals from complex leachates. Additionally, the research activity highlighted that metal sulfide precipitation and biosorption processes have to limit factors that could hinder the process scale-up. Thus, more research is needed to evaluate the environmental impacts of metal recovery from leach liquors.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ahalya, N., Ramachandra, T. V., & Kanamadi, R. D. (2003). Biosorption of heavy metals. Research Journal of Chemistry and Environment, 7(4), 71–79.
Bin, H. U., Yang, T. Z., Liu, W. F., Zhang, D. C., & Lin, C. H. E. N. (2019). Removal of arsenic from acid wastewater via sulfide precipitation and its hydrothermal mineralization stabilization. Transactions of Nonferrous Metals Society of China, 29(11), 2411–2421.
Birungi, Z. S., & Chirwa, E. M. N. (2014). The kinetics of uptake and recovery of lanthanum using freshwater algae as biosorbents: Comparative analysis. Bioresource Technology, 160, 43–51.
Cai, X., Kong, L., Hu, X., & Peng, X. (2021). Recovery of Re (VII) from strongly acidic wastewater using sulphide: Acceleration by UV irradiation and the underlying mechanism. Journal of Hazardous Materials, 416, 126233.
Chassary, P., Vincent, T., Marcano, J. S., Macaskie, L. E., & Guibal, E. (2005). Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy, 76(1), 131–147.
Chen, C., Wen, D., & Wang, J. (2014). Cellular surface characteristics of Saccharomyces cerevisiae before and after Ag (I) biosorption. Bioresource Technology, 156, 380–383.
Choubey, P. K., Dinkar, O. S., Panda, R., Kumari, A., Jha, M. K., & Pathak, D. D. (2021). Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Management, 121, 452–457.
Das, N. (2010). Recovery of precious metals through biosorption—a review. Hydrometallurgy, 103(1), 180–189. https://doi.org/10.1016/j.hydromet.2010.03.016
Das, N., & Das, D. (2013). Recovery of rare earth metals through biosorption: An overview. Journal of Rare Earths, 31(10), 933–943.
Deng, Z., Oraby, E. A., & Eksteen, J. J. (2019). The sulfide precipitation behaviour of Cu and Au from their aqueous alkaline glycinate and cyanide complexes. Separation and Purification Technology, 218, 181–190.
Diniz, V., & Volesky, B. (2005). Biosorption of La, Eu and Yb using Sargassum biomass. Water Research, 39(1), 239–247.
Donia, A. M., Atia, A. A., & Elwakeel, K. Z. (2007). Recovery of gold (III) and silver (I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy, 87(3), 197–206.
Estay, H., Barros, L., & Troncoso, E. (2021). Metal sulfide precipita-tion: Recent breakthroughs and future outlooks. Minerals, 11(12), 1385.
Fomina, M., & Gadd, G. M. (2014). Biosorption: Current perspectives on concept, definition and application. Bioresource Technology, 160, 3–14.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92, 407–418.
Fu, F., Zeng, H., Cai, Q., Qiu, R., Yu, J., & **ong, Y. (2007). Effective removal of coordinated copper from wastewater using a new dithiocarbamate-type supramolecular heavy metal precipitant. Chemosphere, 69, 1783–1789.
Fujiwara, K., Ramesh, A., Maki, T., Hasegawa, H., & Ueda, K. (2007). Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions on l-lysine modified crosslinked chitosan resin. Journal of Hazardous Materials, 146(1), 39–50.
Gadd, G. M. (1993). Interactions of fungip with toxic metals. New Phytologist, 124(1), 25–60.
Gadd, G. (2009). Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. Journal of Chemical Technology and Biotechnology, 84(1), 13–28.
Gao, X., Zhang, Y., & Zhao, Y. (2017). Biosorption and reduction of Au (III) to gold nanoparticles by thiourea modified alginate. Carbohydrate Polymers, 159, 108–115.
Gharabaghi, M., Irannajad, M., & Azadmehr, A. R. (2012). Selective sulphide precipitation of heavy metals from acidic polymetallic aqueous solution by thioacetamide. Industrial and Engineering Chemistry Research, 51(2), 954–963.
Gupta, C. K. (2006). Chemical metallurgy: Principles and practice. John Wiley & Sons.
Hassanien, W. A. G., Desouky, O. A. N., & Hussien, S. S. E. (2014). Bioleaching of some rare earth elements from Egyptian monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak Journal of Science and Technology (WJST), 11(9), 809–823.
Hadjittofi, L., Charalambous, S., & Pashalidis, I. (2016). Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. Journal of Rare Earths, 34(1), 99–104.
Hedrich, S., Joulian, C., Graupner, T., Schippers, A., & Guézennec, A.-G. (2018). Enhanced chalcopyrite dissolution in stirred tank reactors by temperature increase during bioleaching. Hydrometallurgy, 179, 125–131.
Heilmann, M., Jurkowski, W., Buchholz, R., Brueck, T., & Becker, A. M. (2015). Biosorption of neodymium by selected photoautotrophic and heterotrophic species. Journal of Chemical Engineering and Process Technology, 6(4), 1.
Hong, T., Zheng, T., Liu, M., Mumford, K. A., & Stevens, G. W. (2020). Investigation on the recovery of rhenium in the high arsenite wash acid solution from the copper smelting process using reducing sulfide precipitation method. Hydrometallurgy, 195, 105402.
Hussien, S. S. (2014). Biosorption lanthanum pleurotus ostreatus basidiocarp. International Journal of Biomedical Research, 2, 26–36.
Işıldar, A., van Hullebusch, E. D., Lenz, M., Du Laing, G., Marra, A., Cesaro, A., Panda, S., Akcil, A., Kucuker, M. A., & Kuchta, K. (2019). Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)–A review. Journal of Hazardous Materials, 362, 467–481.
Kucuker, M. A. (2018). Biomining concept for recovery of rare earth elements (REEs) from secondary sources. Hamburger Berichte; Bd. 48; Verlag Abfall aktuell der Ingenieurgruppe RUK GmbH, Stuttgart, ISBN 978-3-9817572-8-6.
Kucuker, M. A., & Kuchta, K. (2012). Biosorption with Algae as a green technology for recovery of rare earth metals from E-waste (pp.4–6). Presented at the International conference on Recycling and Reuse.
Kucuker, M. A., & Kuchta, K. (2018). Biomining–biotechnological systems for the extraction and recovery of metals from secondary sources. Global Nest Journal, 20, 737–742.
Kucuker, M. A., Nadal, J. B., & Kuchta, K. (2016). Comparison between batch and continuous reactor systems for biosorption of neodymium (Nd) using microalgae. International Journal of Plant, Animal and Environmental Sciences, 6, 197–203.
Kucuker, M. A., Wieczorek, N., Kuchta, K., & Copty, N. K. (2017). Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS ONE, 12(4), e0175255.
Kumar, M., Nandi, M., & Pakshirajan, K. (2021). Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. Journal of environmental management, 278, 111555.
Kwak, H. W., Yang, Y. S., Kim, M. K., Lee, J. Y., Yun, H., Kim, M. H., & Lee, K. H. (2013). Chromium (VI) adsorption behavior of silk sericin beads. International Journal of Industrial Entomology, 26(1), 47–53.
Lee, C. H., Chen, Y. J., Liao, C. H., Popuri, S. R., Tsai, S. L., & Hung, C. E. (2013). Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metallurgical and Materials Transactions A, 44(13), 5825–5833.
Lewis, A. E. (2010). Review of metal sulfide precipitation. Hydrometallurgy, 104, 222–234.
Lewis, A., & van Hille, R. (2006). An exploration into the sulphide precipitation method and its effect on metal sulphide removal. Hydrometallurgy, 81(3), 197–204.
Li, L., Hu, Q., Zang, J. H., Qi, H. Y., & Zhuang, G. Q. (2011). Resistance and biosorption mechanism of silver ions by Bacillus cereus biomass. Journal of Environmental Sciences, 23(1), 108–111.
Li, H., Oraby, E., & Eksteen, J. (2021). Recovery of copper and the deportment of other base metals from alkaline glycine leachates derived from waste printed circuit boards (WPCBs). Hydrometallurgy, 199, 105540.
Liu, R., Yang, Z., He, Z., Wu, L., Hu, C., Wu, W., & Qu, J. (2016). Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): Inhibitive effects of S (0)-enriched surfaces. Chemical Engineering Journal, 304, 986–992.
Ma, H. W., Liao, X. P., Liu, X., & Shi, B. (2006). Recovery of platinum (IV) and palladium (II) by bayberry tannin immobilized collagen fibre membrane from water solution. Journal of Membrane Science, 278(1), 373–380.
Macingova, E., & Luptakova, A. (2012). Recovery of metals from acid mine drainage. Chemical Engineering, 28, 109–114.
Manahan, S. E. (1990). Hazardous waste chemistry, toxicology, and treatment. CRC Press.
Mao, Y., Hu, H., & Yan, Y. (2011). Biosorption of cesium (I) from aqueous solution by a novel exopolymers secreted from Pseudomonas fluorescens C-2: Equilibrium and kinetic studies. Journal of Environmental Sciences, 23(7), 1104–1112.
Marra, A. (2017). Innovative treatments for resource recovery from waste electrical and electronic equipment (PhD thesis), Università degli Studi di Salerno, Italy.
Mata, Y. N., Torres, E., Blazquez, M. L., Ballester, A., González, F. M. J. A., & Munoz, J. A. (2009). Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. Journal of Hazardous Materials, 166(2), 612–618.
Michalak, I., Chojnacka, C., & Witek-Krowiak, A. (2013). State of the art for the biosorption process—A review. Applied Biochemistry and Biotechnology, 170(6), 1389–1416.
Migdisov, A. A., Williams-Jones, A. E., Lakshtanov, L. Z., & Alekhin, Y. V. (2002). Estimates of the second dissociation constant of H2S from the surface sulfidation of crystalline sulfur. Geochimica Et Cosmochimica Acta, 66(10), 1713–1725.
Mokone, T. P., Van Hille, R. P., & Lewis, A. E. (2009). Mechanisms responsible for particle formation during metal sulphide precipitation processes. Water institute of Southern Africa and international mine water association. Pretoria.
Muñoz, A. J., Espínola, F., & Ruiz, E. (2017). Biosorption of Ag (I) from aqueous solutions by Klebsiella sp. 3S1. Journal of Hazardous Materials, 329, 166–177.
Oliveira, R. C., & Garcia, O. (2009). Study of biosorption of rare earth metals (La, Nd, Eu, Gd) by Sargassum sp. biomass in batch systems: Physicochemical evaluation of kinetics and adsorption models. In Advanced materials research (Vol. 71, pp. 605–608). Trans Tech Publications.
Oliveira, R. C., Jouannin, C., Guibal, E., & Garcia, O. (2011). Samarium (III) and praseodymium (III) biosorption on Sargassum sp.: batch study. Process biochemistry, 46(3), 736–744.
Panda, S., Costa, R. B., Shah, S. S., Mishra, S., Bevilaqua, D., & Akcil, A. (2021). Biotechnological trends and market impact on the recovery of rare earth elements from bauxite residue (red mud)–A review. Resources, Conservation and Recycling, 171, 105645.
Panda, S., Parhi, P. K., Pradhan, N., Mohapatra, U. B., Sukla, L. B., & Park, K. H. (2012) Extraction of copper from bacterial leach liquor of a low grade chalcopyrite test heap using LIX 984N-C”. Hydrometallurgy (Elsevier) (Vol. 121–124, pp. 116–119). https://doi.org/10.1016/j.hydromet.2012.03.008
Pohl, A. (2020). Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water, Air, and Soil Pollution, 231(10), 1–17.
Prodromou, M., & Pashalidis, I. (2016). Europium adsorption by non-treated and chemically modified opuntia ficus indica cactus fibres in aqueous solutions. Desalination and Water Treatment, 57(11), 5079–5088.
Robalds, A., Naja, G. M., & Klavins, M. (2016). Highlighting inconsistencies regarding metal biosorption. Journal of Hazardous Materials, 304, 553–556.
Sahan, M., Kucuker, M. A., Demirel, B., Kuchta, K., & Hursthouse, A. (2019). Determination of metal content of waste mobile phones and estimation of their recovery potential in Turkey. International Journal of Environmental Research and Public Health, 16(5), 887.
Sari, A., Mendil, D., Tuzen, M., & Soylak, M. (2009). Biosorption of palladium (II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. Journal of Hazardous Materials, 162(2), 874–879.
Sert, S., Kütahyali, C., Inan, S., Talip, Z., Cetinkaya, B., & Eral, M. (2008). Biosorption of lanthanum and cerium from aqueous solutions by Platanus orientalis leaf powder. Hydrometallurgy, 90(1), 13–18.
Sethurajan, M. (2016). Metallurgical sludges, bio/leaching and heavy metals recovery (Zn, Cu) (Doctoral dissertation, Paris Est).
Sethurajan, M., van Hullebusch, E. D., Fontana, D., Akcil, A., Deveci, H., Batinic, B., Leal, J. P., Gasche, T. A., Kucuker, M. A., Kuchta, K., Neto, I. F. F., Soares, H. M. V. M., & Neto, A. I. F. (2019). Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Critical Reviews in Environmental Science and Technology, 1–64.
Talaro, K. P., & Talaro, A. (2002). Physical and chemical control of microbes. In Foundations in Microbiology (pp. 325–326). U.S. Mineral Commodity Summaries (2015).
Tewari, N., Vasudevan, P., & Guha, B. K. (2005). Study on biosorption of Cr(VI) by Mucor hiemalis. Biochemical Engineering Journal, 23(2), 185–192.
Thomas, M., Białecka, B., & Zdebik, D. (2014). Sources of copper ions and selected methods of their removal from wastewater from the printed circuits board production. Inzynieria Ekologiczna, 37, 31–49.
Torab-Mostaedi, M., Asadollahzadeh, M., Hemmati, A., & Khosravi, A. (2015). Biosorption of lanthanum and cerium from aqueous solutions by grapefruit peel: Equilibrium, kinetic and thermodynamic studies. Research on Chemical Intermediates, 41(2), 559–573.
Tuncuk, A., Stazi, V., Akcil, A., Yazici, E. Y., & Deveci, H. (2012). Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Minerals Engineering, 25(1), 28–37. https://doi.org/10.1016/j.mineng.2011.09.019
Uçar, D. (2017). Sequential precipitation of heavy metals using sulfide-laden bioreactor effluent in a pH controlled system. Mineral Processing and Extractive Metallurgy Review, 38(3), 162–167.
Varsihini, C. J. S., Das, D., Das, N. (2014). Optimization of parameters for Cerium(III) biosorption onto biowaste materials of animal and plant origin using 5- level Box–Behnken design: equilibrium, kinetic, thermodynamic and regeneration studies. Journal of Rare Earths, 32(8), 745–758.
Vemic, M., Bordas, F., Comte, S., Guibaud, G., Lens, P. N. L., & Van Hullebusch, E. D. (2016). Recovery of molybdenum, nickel and cobalt by precipitation from the acidic leachate of a mineral sludge. Environmental Technology, 37(17), 2231–2242.
Vijayaraghavan, K. (2015). Biosorption of lanthanide (praseodymium) using Ulva lactuca: Mechanistic study and application of two, three, four and five parameter isotherm models. Journal of Environment and Biotechnology Ressearch, 1(1), 10–17.
Vijayaraghavan, K., & Jegan, J. (2015). Entrapment of brown seaweeds (Turbinaria conoides and Sargassum wightii) in polysulfone matrices for the removal of praseodymium ions from aqueous solutions. Journal of Rare Earths, 33(11), 1196–1203.
Vijayaraghavan, K., & Yun, Y. S. (2008). Bacterial biosorbents and biosorption. Biotechnology Advances, 26(3), 266–291. https://doi.org/10.1016/j.biotechadv.2008.02.002
Volesky, B. (1987). Biosorbents for metal recovery. Trends in Biotechnology, 5(4), 96–101.
Volesky, B. (2003). Biosorption process simulation tools. Hydrometallurgy, 71(1), 179–190.
Volesky, B. (2007). Biosorption and me. Water Research, 41(18), 4017–4029.
Wang, J., & Chen, C. (2009). Biosorbents for heavy metals removal and their future. Biotechnology Advances, 27(2), 195–226.
Wang, L. K., Yung-tse, H., & Shammas, N. K. (Eds.). (2005). Physicochemical treatment processes (Vol. 3). Humana Press.
Wang, W., Pranolo, Y., & Cheng, C. Y. (2011). Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy, 108(1), 100–108.
Zhang, Y., Feng, X., & **, B. (2020). An effective separation process of arsenic, lead, and zinc from high arsenic-containing copper smelting ashes by Alkali leaching followed by sulfide precipitation. Waste Management and Research, 38(11), 1214–1221.
Zhu, X., Li, W., Tang, S., Zeng, M., Bai, P., & Chen, L. (2017). Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere, 175, 365–372.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kucuker, M.A. (2024). Recovery of Metals from Leach Liquors: Biosorption versus Metal Sulfide Precipitation. In: Panda, S., Mishra, S., Akcil, A., Van Hullebusch, E.D. (eds) Biotechnological Innovations in the Mineral-Metal Industry. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-43625-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-43625-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-43624-6
Online ISBN: 978-3-031-43625-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)