Abstract
The interaction between the return function of the circulation and cardiac function was described by Guyton. In doing so, Guyton developed a model incorporating the works of Starling and Frank with his own work. The Guytonian model provides a simple framework for understanding the possible effects of fluid boluses or vasoactive medications on various macro-hemodynamic parameters. In this chapter, we describe briefly the Guytonian model of circulation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Mean systemic pressure
- Right atrial pressure
- Resistance to venous return
- Venous return
- Venous return curve
- Cardiac output
- Cardiac function curve
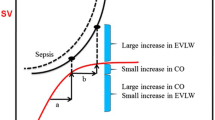
In this chapter, the different hemodynamic principles from Frank–Starling to Guyton–Hall will be discussed. However, it were Sydney Patterson (1882–1960) and Ernest Starling (1866–1927) that first described the mechanical factors that determine the output of both ventricles [1]. Before Patterson, it was Otto Frank (1865–1944) who continued the experiments at Carl Ludwig’s Physiological Institute. He looked at an improved frog heart preparation from the viewpoint of skeletal muscle mechanics, substituting volume and pressure for length and tension, which enabled him to measure isovolumetric and isotonic contractions. With increasing filling of the frog ventricle, diastolic pressure was elevated at each step. However, beyond a certain filling pressure, it decreased. Otto Frank compiled all of the data in the famous pressure–volume diagram [2]. Afterwards, Arthur Guyton (1919–2003) credited Ernest Starling for appreciating that output from the heart is dependent upon the return of venous blood and that venous return is dependent upon the pressure upstream to the heart in the systemic circulation, which Starling called mean systemic pressure. Starling had not dealt with the mechanics of the systemic circulation or the factors that determine flow back to the heart; the concept of venous return and its determinants awaited Guyton: “When a change occurs in the hemodynamics of the circulatory system one cannot predict what will happen to the cardiac output unless he takes into consideration both the effect of this change on the ability of the heart to pump blood and also the tendency for blood to return to the heart from the blood vessels” [3]. Together with John Hall, he wrote the famous Guyton and Hall Textbook of Medical Physiology. Going further back in time, William Harvey (1578–1657) was an English physician who made seminal contributions to anatomy and physiology. He was the first known physician to describe completely and in detail the systemic circulation and properties of blood being pumped to the brain and body by the heart. Stephen Hales (1677–1761) was an English clergyman who made major contributions to a range of scientific fields including botany, pneumatic chemistry and physiology. He was the first person to measure blood pressure and he invented several devices, including a ventilator. We need to understand that pulse pressure is more important than mean arterial pressure (MAP) as flow needs to bring oxygen to the tissues. On the other hand, in patients with advanced decompensated heart failure (but also in sepsis) we already know that central venous pressure (CVP) is more important than cardiac output (CO) in explaining worsening renal function (WRF) (Fig. 4.1) [4, 5].
Statistical model of nonparametric logistic regression showing the relationship between mean central venous pressure during the first 24 h after admission and the probability of new or persistent acute kidney injury. Note the plateau for the incidence of acute kidney injury (AKI) when the lower limit of central venous pressure (CVP) was between 8 and 12 mmHg. Over this limit, the rise in CVP was associated with a sharp increase in new or persistent AKI incidence. Adapted from Legrand et al. [5]
However, the microcirculation is equally important as it manages bodily fluids. The endothelial glycocalyx (EG) is a thin-walled layer that keeps fluids in place and only limited filtration occurs (at the venous side). The revised Starling principle developed by Charles Michel and Sheldon Weinbaum has gained importance, and we know that diffusion and convection are two different things as are coherence and heterogeneity [6, 7]. The physiological compartment that manages fluids in the body is the microcirculation. That is why titrating IV fluids based on macrocirculatory parameters can lead to inappropriate administration of fluids leading to overload and organ dysfunction. In order to preserve organ and microcirculatory function, we should therefore limit fluid intake and avoid fluid accumulation. Besides giving a fluid bolus to increase circulating volume and to improve CO, the use of early vasopressors can help to convert unstressed to stressed volume, but this may not be sufficient in patients with profound capillary leak and vasodilation. A rapid fluid bolus can potentially improve hemodynamic parameters during shock states; however, too rapid infusion may cause harm to the EG layer and too much fluid may cause venous congestion and significant morbidity and mortality as previously stated. Fluids are drugs and should only be given when the patient is a fluid responder (i.e. both ventricles acting on the steep part of Frank–Starling curve) and when needed (i.e. shock state with increased lactate). They should never be used to treat or improve the “numbers” (e.g. low MAP, CO, CVP or urine output) (Fig. 4.2).
Suggested Reading
-
1.
Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol 1914;48(5): 357–79.
-
2.
Zimmer HG. Who discovered the Frank-Starling mechanism? News Physiol Sci 2002;17:181-84.
-
3.
Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 1957;189(3):609-15.
-
4.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013;62(6):485-95.
-
5.
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Critical Care 2013;17(6):R278.
-
6.
Michel CC, Woodcock TE, Curry FE: Understanding and extending the Starling principle. Acta Anaesthesiol Scand 2020;64(8):1032-7.
-
7.
Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol 2004;557(Pt 3):889-907.
Illustration of the concept of preload dependence. (a) Fluid responsiveness illustrated by a greater increase in mean systemic filling pressure with 7 mmHg (from 22 to 29 mmHg) compared to the 2 mmHg increase in CVP (from 6 to 8 mmHg) resulting in a 15% increase in cardiac output from 5.4 to 6.2 L/min. (b) Fluid unresponsiveness illustrated by an equal increase in mean systemic filling pressure with 4 mmHg (from 24 to 28 mmHg) and a 3 mmHg increase in CVP (from 8 to 11 mmHg) not resulting in a significant increase in cardiac output (from 5.9 to 6.0 L/min)
The learning objectives of this chapter are:
-
1.
To learn about various factors regulating venous return.
-
2.
To understand the possible effects of fluid boluses and vasoactive drugs on venous return.
-
3.
To learn about cardiac function curve and factors contributing to the shape of the curve.
-
4.
To understand the effects of fluid boluses and vasoactive drugs on cardiac function curves and cardiac output.
-
5.
To understand the framework provided by Guyton in understanding the circulation of blood and the effects (and possible harmful effects) of fluid boluses on the macro-hemodynamic parameters.
Mr. X, a 67-year-old gentleman with a history of long-standing diabetes mellitus and benign prostatic hypertrophy, presented to the emergency department (ED) with fever and dysuria for the past 3 days. His wife noticed him to be disoriented since morning and decided to bring him to the ED. On examination, he was drowsy but rousable. His extremities were cold to touch. He had a heart rate of 116/min, blood pressure of 70 mmHg systolic and respiratory rate of 24/min. Pulse oximetry reading showed 95% while breathing room air. Capillary refill time was 6 seconds. Systemic examination findings were unremarkable except for some tenderness on deep palpation of the right flank. The emergency physician decided to infuse him rapidly with 500 ml of Ringer’s lactate.
Questions
-
Q1. What are the possible hemodynamic effects of this fluid bolus on Mr. X?
Introduction
The purpose of resuscitation in a shocked patient is to maintain the perfusion of tissues and organs. Options available for resuscitation are basically limited to intravenous fluids, vasoactive agents (vasopressors and inotropes) and blood transfusion (in specific situations). A detailed understanding of the effects of these agents on circulation is important for their appropriate use. Numerous scientists have contributed to our current understanding of circulatory physiology [1]. Prominent among them were the German physiologist Otto Frank and British physiologist Earnest Henry Starling. They are credited with describing the relationship between the length of cardiac muscle just before cardiac contraction and the strength of the contraction itself (Frank–Starling law). American physiologist, Arthur C Guyton, went a step further and described the dynamics of blood in circulatory physiology. His model of circulation integrated two different functions “the return function” and “the cardiac function” previously described separately by various researchers. The Guytonian model of circulation is now the most widely followed model of circulatory physiology and is described in detail in Guyton and Hall’s Textbook of Physiology [2]. In this chapter, we shall reappraise the circulatory physiology as proposed by Guyton and understand the possible effects of fluid and vasoactive agents on circulation. See also Chap. 5 to understand heart-lung interactions and fluid responsiveness.
What Are the Factors That Determine Flow of Blood from Peripheral Circulation to Heart?
Hagen–Poiseuille’s law states that the flow of fluid through a system is related to the pressure gradient between two parts of the system (difference between upstream pressure and downstream pressure) divided by the resistance to flow (analogous to Ohm’s law of electrical current flow).
Bayliss and Starling first proposed the role of peripheral circulation in determining the return of blood to the heart (“venous return”). They coined the term mean circulatory filling pressure (Pmcf), described as the average pressure in the circulation when the heart is stopped momentarily and the pressure in the entire circulatory system equilibrates, for example after administration of the cardioplegic solution. [3] A closely related and more widely used term is mean systemic pressure (Pms) defined as the equilibrium pressure only in the systemic circulation in the absence of any flow, ignoring the heart and pulmonary circulation (e.g. by clam** the aorta and venae cavae). Bayliss and Starling also realized that during active flow, Pms (or Pmcf) is determined primarily by the pressure in the venous side of the circulation as the larger volume of blood is stored in the high-capacitance venous system. Years later, Guyton proposed Pms as the upstream pressure for the venous return [4]. He described the right atrial pressure (RAP) as the downstream pressure with the difference between Pms and RAP as the net driving pressure for venous return to the heart. Following the Hagen–Poiseuille’s law, the relationship between VR, Pms, RAP and RVR can be plotted as follows:
VR venous return, Pms mean systemic pressure, RAP right atrial pressure, RVR resistance to venous return
What Are the Factors That Determine Mean Systemic Pressure?
Pms is the result of elastic recoil potential, stored in the walls of the components of the circulatory system and is determined by the volume of blood that stretches the vessels further beyond their normal shape (so-called stressed volume). Stressed volume, in turn, depends on two factors—total circulatory volume and the capacitance of the circulatory system. Normally, only 30% of the blood volume (mostly in the venous circulation) contributes to the stressed volume. The rest of the blood volume (so-called unstressed volume) does not contribute to the circulation but acts only as a reserve. There can be only two ways to increase “stressed volume” (and Pms in this process), either by increasing circulatory volume (e.g. by fluid loading) or by decreasing venous capacitance (e.g. by increasing sympathetic tone with norepinephrine or other vasopressors).
Under normal circumstances, the blood volume remains near constant. Thus, the major factor that determines the return of blood to the heart is resistance to venous return, produced as a result of changes in resistance of blood vessels at the level of organs [2]. The change in resistance and venous capacitance is determined by local factors. For example, during states of high oxygen demand at the organ level, local vasodilator substances are released, decreasing the overall resistance to venous return. Another important factor that determines venous return in the physiological state is RAP, the downstream pressure for VR. Appropriate gradient to VR is maintained by kee** the RAP closer to 0 mmHg by cardiac action [2].
Guyton’s Experiment and Venous Return Curve
In the classical animal experiment, Guyton and colleagues cannulated the right atrium and pulmonary artery of anaesthetized dogs and drained the right atrial blood directly to the pulmonary artery via a horizontal thin rubber tubing bypassing the right ventricle [5]. Blood was pumped from the right atrium to the pulmonary artery by using an artificial pump. The pump speed was maintained sufficiently to keep the rubber tubing in a semi-closed state. Pressure at the beginning of the perfusion circuit (reflecting right atrial pressure) was varied by adjusting the height of the horizontal rubber tubing. From this model, they could demonstrate the effects of varying right atrial pressure (from very high positive to very low negative) on venous return (quantified by the amount of blood flowing to the pulmonary artery). They graphically represented their findings with RAP plotted on the x-axis and VR on the y-axis as shown in Fig. 4.3
As can be seen from the extreme left curve in Fig. 4.3, with progressive lowering of RAP, VR increases until a point beyond which it remains in a plateau state at all RAP values more negative than −2 to −4 mmHg. Guyton demonstrated that this plateau is produced because of the progressive collapse of great veins, due to higher surrounding pressure. In the intersection between VR curve with X-axis, the RAP reaches the value of Pms and the VR becomes zero (thankfully only in theory!). The slope of the curve is related to −1/RVR, i.e. steeper venous return curve means a decrease in resistance to VR.
Starling’s Experiment
In anaesthetized dogs, Starling and his colleagues ligated the inferior vena cava, distal aorta and branches of the aortic arch, kee** the pulmonary circulation and blood flow to the heart itself intact [6]. Through an aortic cannula, systemic blood flow was diverted via the extracorporeal circuit into an elevated reservoir. Blood was returned back to the right atrium through a cannula placed in the superior vena cava. The rate of blood flow from the reservoir into the right atrium was adjusted using a resistor. Over a wide range, the heart could eject whatever volume of blood the system returned to the right atrium. With the increasing the return of blood into the right atrium (by adjusting the Starling resistor), there was a slow rise in right atrial pressure up to a certain limit. Beyond that limit, the rise in RAP was abrupt, limiting the further return of blood to the right atrium. Starling graphically displayed RAP on the y-axis and the return of blood to the right atrium (VR) on the x-axis [6]. Starling’s original series of curves are shown in Fig. 4.4.
Original starling curve. Relationship between right atrial pressure and venous return in different animals. Permission required [6]
Effect of Fluid Bolus on Venous Return Curve
A fluid bolus can have three potential effects on VR.
-
1.
Fluid bolus is expected to shift the VR curve to the right by increasing the “stressed volume” (and Pms) (as can be seen in the extreme right curve in Fig. 4.3). The increase in Pms in turn is expected to increase the gradient (Pms–RAP) for VR. In reality, the relationship between fluid bolus and an increase in Pms is not as simple and depends on venous capacitance. Another factor that plays a significant role in determining the rise in Pms after fluid bolus is the extent of capillary leak in various disease states. In cases of profound vasoplegia (as in septic shock), venous capacitance and capillary leak increase significantly and fluid bolus may fail to increase “stressed volume”.
-
2.
The increase in circulatory volume is also expected to shift the VR curve clockwise, resulting in a decrease in RVR. This drop in RVR can also facilitate venous return (as seen in the middle curve in Fig. 4.3).
-
3.
The rise in venous return in turn will produce a rise in RAP. Up to a certain limit, the rise in RAP is minimal with a normally contractile heart, but beyond that limit heart cannot accommodate blood further and RAP starts rising disproportionately, resulting in a fall in VR [6].
Cardiac Function Curve
The amount of blood ejected by the ventricle in a single cardiac cycle is called the stroke volume. The critical determinant of stroke volume is the ventricular volume (and pressure), just before the onset of ventricular contraction or at end diastole. This end-diastolic volume (or pressure) is also known as ventricular preload. At the molecular level, preload is determined by the length of the sarcomere at the end of diastole. This relationship was discovered independently by both Otto Frank and Ernest Henry Starling, while working on isolated heart–lung preparation in the animal model (frog and dog, respectively) [1]. Other factors that determine the stroke volume are the load ventricle faces during the 1ejection of blood (also known as afterload) and the elastance of ventricle (or cardiac contractility). The amount of blood ejected by the ventricle in one minute is known as cardiac output and is determined by the stroke volume and heart rate.
Guyton graphically described this relationship between cardiac output and preload (also known as the Frank–Starling relationship), depicting cardiac output on the x-axis and the right atrial pressure (as the surrogate of end-diastolic pressure) on the y-axis producing a curvilinear pattern (cardiac function curve). Figure 4.5 below shows a series of cardiac function curves.
As can be seen in Fig. 4.5, the cardiac function curve reaches a plateau beyond a certain point (curve 1). Another important point to note is that the cardiac function curve is not a single curve but a series of curves and depends on the afterload and contractility of the ventricle. With a decrease in afterload or an increase in contractility, the cardiac function curve is shifted upwards and towards the left (curve 2 in Fig. 4.5). On the contrary, an increase in afterload and a decrease in contractility shift the curve towards the right and downwards (curve 3 in Fig. 4.5).
Integrating the Return Function with Cardiac Function
Guyton and colleague proposed a framework to show how the return function of the circulation and cardiac function operate together in the overall circulatory system [7]. Two basic functions of the heart play a significant role in the circulatory dynamics. Firstly, in the steady state, the heart pumps out whatever comes in, i.e. VR must be equal to cardiac output (CO). Secondly, VR is facilitated by the constant pum** of blood from the right heart that keeps the RAP low. As VR and CO must be equal in steady state and both VR curve and cardiac function curve use RAP as the independent variable, the two curves can be superimposed, as seen in Fig. 4.6. Intersection of these two curves is the equilibrium point where VR is equal to CO. This equilibrium point defines the VR/CO in different clinical situations and will be discussed further in the next section.
Overall Effect of Fluid Bolus on Circulation
Figure 4.7 graphically displays the overall effects of fluid bolus and vasoactive drugs.
Integrated cardiac function and venous return curves. RAP right atrial pressure, CO cardiac output, VR venous return. Dotted line = venous return curve at baseline. Dashed line = venous return curve shifted to right after fluid bolus. Curved lines = different cardiac function curves. Points A–F = described in detail in the text
As discussed earlier, intravenous fluid infusion raises the Pms by increasing stressed volume (unless there is an extreme vasoplegia or capillary leak) and shifts the VR curve to the right. This produces a new equilibrium point in the integrated venous return and cardiac function curve. As can be seen in Fig. 4.7, following a fluid bolus, the venous return curve is shifted from the baseline (dotted line) towards the right (dashed line). This results in a shifting of the equilibrium point from point A to point B with a corresponding increase in cardiac output, provided that the new equilibrium point (point B) is in the steep part of the cardiac function curve (permissive heart; middle cardiac function curve). Fluid bolus may also increase the venous return by shifting the curve clockwise (by reducing the resistance to venous return; not shown in Fig. 4.7) [8]. Another relatively less important effect of fluid bolus is a decrease in afterload by haemodilution (and reduction in viscosity of the blood). A decrease in afterload shifts the cardiac function curve towards the left (not shown in Fig. 4.7) [6].
In cases of a poorly contractile heart (when the cardiac function curve is shifted rightward), with similar change in VR, there is minimal or no change in CO and a disproportionate rise in RAP (point C to point D in Fig. 4.7). In addition to the impediment of VR to the heart (by decreasing the pressure gradient for VR), high RAP produces back pressure changes. Raised RAP can produce further reduction in organ perfusion by increasing renal, hepatic and intestinal venous pressure and also impairment in microcirculatory flow [9].
Inotropic infusion or a decrease in afterload can shift the cardiac function curve to the left. With this changed cardiac function curve, an increase in Pms (and VR) will increase CO further (point E to point F in Fig. 4.7).
Validation of Guytonian Model in Human Studies
Maas and colleagues tested the feasibility of measuring Pms in intact circulation and tested the effects of hypo- and hypervolemia on Pms [10]. In a study on postoperative cardiac surgery patients, they estimated the VR curve by constructing a regression line between pairs of cardiac output (as surrogate of VR) and central venous pressure (CVP as a surrogate of RAP). CVP and CO values are measured during 12-second inspiratory hold manoeuvre at four different levels of plateau pressure (5, 15, 25 and 35 cm H2O). CO was measured using pulse contour analysis. The intercept of the regression line at the x-axis was taken as Pms (as discussed in an earlier section). VR curves were constructed at baseline (in resting state), during relative hypovolemia (by raising the head of the bed at 30°) and after hypervolemia (by infusion of 500 ml colloids). The study could confirm that Pms decreases with hypovolemia and increases with hypervolemia [10].
In another study on postoperative cardiac surgery patients, the same group tested the effects of norepinephrine on CO and VR curves [11]. In all patients, norepinephrine caused an increase in Pms, resistance to VR and systemic vascular resistance (SVR). However, the effect of norepinephrine on cardiac output was variable. Patients who had a decrease in CO on norepinephrine also had a significantly higher rise in CVP, RVR and SVR compared to those who had an increase in CO on norepinephrine [11]. Persichini and colleagues observed similar effects of norepinephrine on Pms and VR in human septic shock patients [12].
Cecconi and colleagues tested the effects of fluid challenge on Pmsa (a Pms analogue) and VR gradient (dVR = Pmsa – CVP) using Navigator™ technology [13]. Studying postoperative patients, they found that the increase in cardiac output by >10% (“responders”) with fluid challenge was associated with the corresponding increase in dVR. In “non-responders”, in turn, dVR did not increase despite the consistent increase in Pms, because of a disproportionate rise in CVP [13]. These findings validate the Guytonian model of circulation.
Case Vignette
Mr. X, the patient in the vignette, has clinical evidence of septic shock and the purpose of administering a fluid bolus (500 ml Ringer’s lactate in this vignette) is to improve tissue perfusion by increasing the cardiac output.
However, the effects of fluid boluses on cardiac output are variable based on the underlying pre-dominant macro-hemodynamic state. Three possible hemodynamic effects of this fluid bolus on Mr X are as follows:
-
Scenario 1: Pms may actually rise with minimal rise in RAP, thus increasing VR and CO and in turn improving the organ perfusion (desirable effect).
-
Scenario 2: In patients with extreme vasoplegia, stressed volume and Pms may not increase and both venous return and cardiac output remain unchanged. This will harm Mr. X in the long run by producing a positive cumulative fluid balance.
-
Scenario 3: There may be a minimal rise in Pms with a disproportionate increase in RAP and no change in VR or CO. The rise in RAP may in turn reduce organ perfusion further (undesirable effect).
Patients who increase their cardiac output by at least 10% after a rapid bolus of intravenous fluid are described as fluid responders (as in scenario 1). Patients who fall in scenarios 2 and 3 are called fluid non-responders. Fluid responsiveness can be detected by actually challenging the patient with a defined fluid bolus quickly or by performing certain clinical manoeuvres to look for it without actually giving fluid.
Conclusion
The effects of fluid on hemodynamic parameters are not straightforward and depend on various underlying patient-related factors. To obtain a desirable outcome of fluid bolus, it is important for the clinician to be reasonably confident about possible increase in stroke volume (and cardiac output) post-bolus administration. The later can be achieved by careful clinical examination supported by appropriate tests of fluid responsiveness or a carefully performed fluid challenge. While administering fluid boluses, one also should not forget the possible harmful consequences of a fluid bolus, especially when there is no improvement in stroke volume (or cardiac output).
Take-Home Messages
-
Rapid fluid bolus can potentially improve hemodynamic parameters in a shock state provided several patient-related conditions are fulfilled.
-
For an increase in stroke volume (or cardiac output), stressed volume (and mean systemic pressure) should increase significantly with only minimal change in right atrial pressure. Also, both ventricles must be working in the steep portion of the cardiac function curve.
-
The increase in stressed volume may not be sufficient in profound vasodilatory state and in the presence of leaky capillaries.
-
When it does not produce improvement in macro-hemodynamic parameters, fluid boluses can only contribute to fluid overload and potential harm associated with the same.
-
Potential harms associated with fluid bolus cannot be ignored and need to be considered before prescription.
References
Katz AM. Ernest Henry Starling, his predecessors, and the “law of the heart”. Circulation. 2002;106:2986–92.
Hall JE. Guyton and Hall textbook of medical physiology. 13th ed. Philadelphia: Elsevier; 2016.
Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16:159–318.
Guyton AC, Lindsey AW, Kaufmann BN. Effect of mean circulatory filling pressure and other peripheral circulatory factors on cardiac output. Am J Phys. 1955;180:463–8.
Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Phys. 1957;189:609–15.
Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48:357–79.
Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46.
Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock—part I: physiology. Crit Care Med. 2013;41:255–62.
Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21.
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med. 2009;37:912–8.
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41:143–50.
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, Monnet X. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med. 2012;40:3146–53.
Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, Rhodes A. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–305.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Ghosh, S. (2024). Fluid Dynamics During Resuscitation: From Frank–Starling to the Reappraisal of Guyton. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)