Summary
Since the time of years following World War II, tuberculosis (TB) has become a treatable disease due to the discovery of antibiotics such as streptomycin. But now, TB has poised to make a dramatic and deadly comeback again owing to the emergence of some drug-resistant (DR) strains, including multidrug-resistant (MDR), extensively drug-resistant (XDR), and totally drug-resistant (TDR) strains. Health professionals are alarmed that these TB strains are so virulent that they are called “virtually untreatable” even with the most potent anti-TB drugs available. Inappropriate TB practice conducted, mainly in develo** countries, is considered the main reason behind DR. These malpractices include inadequate treatment of TB patients due to incorrect drug combinations, insufficient dose irregularities, or poor adherence. Consequently, both public and non-public sectors contribute to DR-TB. The current chapter sheds light on various DR mechanisms that hinder effective treatment with existing anti-TB drugs. Multiple strategies to overcome the problem of DR-TB have also been discussed. The chapter provides recent updates on newly developed drugs, clinical trials in the pipeline, and international recommendations to manage resistance. This information is essential for develo** a more effective therapy that is potent against DR-TB and can shorten the antibiotic course required for both drug-susceptible TB and DR-TB.
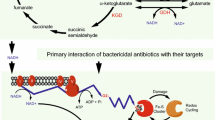
Graphical Abstract

Mechanisms of resistance to anti-tubercular drugs and their possible modulation
Science has no idea how much it owns the imagination.
Ralph Emerson
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kresge N, Simoni RD, Hill RL (2004) Selman Waksman: the father of antibiotics. J Biol Chem 279(48):e7
Waksman SA, Lechevalier HA (1949) Neomycin, a new antibiotic active against streptomycin-resistant bacteria, including tuberculosis organisms. Science 109(2830):305–307
Society AT (1992) Control of tuberculosis in the United States. Am Rev Respir Dis 146:1623–1633
Grosset JH (1989) Present status of chemotherapy for tuberculosis. Rev Infect Dis 11(Suppl 2):S347–S352
Hopewell P (1994) The cure: organization and administration of therapy for tuberculosis. In: Tuberculosis: back to the future. Wiley, Chichester, pp 99–120
Iseman MD, Cohn DL, Sbarbaro JA (1993) Directly observed treatment of tuberculosis—we can’t afford not to try it. Mass Med Soc
Iseman MD (1994) Evolution of drug-resistant tuberculosis: a tale of two species. Proc Natl Acad Sci 91(7):2428–2429
CfD C (1991) Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons–Florida and New York, 1988–1991. MMWR Morb Mortal Wkly Rep 40(34):585
Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW (1993) The emergence of drug-resistant tuberculosis in New York City. N Engl J Med 328(8):521–526
Shah NS, Wright A, Bai G-H, Barrera L, Boulahbal F, Martín-Casabona N, Drobniewski F, Gilpin C, Havelková M, Lepe R: Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis 13(3):380
Verma H, Choudhary S, Singh PK, Kashyap A, Silakari O (2019) Decoding the signature of molecular mechanism involved in mutation associated resistance to 1,3-benzothiazin-4-ones (Btzs) based DprE1 inhibitors using BTZ043 as a reference drug. Mol Simul 45(18):1515–1523
Velayati AA, Farnia P, Masjedi MR (2013) The totally drug resistant tuberculosis (TDR-TB). Int J Clin Exp Med 6(4):307
Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, ZiaZarifi AH, Hoffner SE (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136(2):420–425
Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, Van Soolingen D, Jensen P, Bayona J (2010) Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375(9728):1830–1843
Jain A, Mondal R (2008) Extensively drug-resistant tuberculosis: current challenges and threats. FEMS Immunol Med Microbiol 53(2):145–150
Annabel B, Anna D, Hannah M (2019) Global tuberculosis report 2019. World Health Organization, Geneva
Ahmad S, Mokaddas E (2014) Current status and future trends in the diagnosis and treatment of drug-susceptible and multidrug-resistant tuberculosis. J Infect Public Health 7(2):75–91
Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD (2011) The social determinants of tuberculosis: from evidence to action. Am J Public Health 101(4):654–662
Smith T, Wolff KA, Nguyen L (2012) Molecular biology of drug resistance in Mycobacterium tuberculosis. In: Pathogenesis of Mycobacterium tuberculosis and its interaction with the host organism. Springer, pp 53–80
Sharma S, Kumar M, Sharma S, Nargotra A, Koul S, Khan IA (2010) Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicrob Chemother 65(8):1694–1701
Hugonnet J-E, Blanchard JS (2007) Irreversible inhibition of the Mycobacterium tuberculosis β-lactamase by clavulanate. Biochemistry 46(43):11998–12004
Meena S, Shivangi ML (2018) Interaction of Mycobacterium tuberculosis H37Rv with microfold cell leads to a New Era of infection in host. Ann Clin Lab Res 6(3):246
Nikaido H, Brennan P (1995) The envelope of mycobacteria. Annu Rev Biochem 64:29–63
Liu J, Rosenberg EY, Nikaido H (1995) Fluidity of the lipid domain of cell wall from Mycobacterium chelonae. Proc Natl Acad Sci 92(24):11254–11258
Vandal OH, Nathan CF, Ehrt S (2009) Acid resistance in Mycobacterium tuberculosis. J Bacteriol 191(15):4714–4721
Raynaud C, Papavinasasundaram K, Speight RA, Springer B, Sander P, Böttger EC, Colston MJ, Draper P (2002) The functions of OmpATb, a pore-forming protein of Mycobacterium tuberculosis. Mol Microbiol 46(1):191–201
Sequoia Ecosystem and Recreation Preserve Act of 1999 (1999) 106th Congress edn
Dye C, Williams BG (2010) The population dynamics and control of tuberculosis. Science 328(5980):856–861
Timmins GS, Deretic V (2006) Mechanisms of action of isoniazid. Mol Microbiol 62(5):1220–1227
Aslan G, Tezcan S, Serin MS, Emekdas G (2008) Genotypic analysis of isoniazid and rifampin resistance in drug-resistant clinical Mycobacterium tuberculosis complex isolates in southern Turkey. Jpn J Infect Dis 61(4):255–260
Mokrousov I, Narvskaya O, Otten T, Limeschenko E, Steklova L, Vyshnevskiy B (2002) High prevalence of KatG Ser315Thr substitution among isoniazid-resistant Mycobacterium tuberculosis clinical isolates from northwestern Russia, 1996 to 2001. Antimicrob Agents Chemother 46(5):1417–1424
Zhang Y, Yew W (2015) Mechanisms of drug resistance in Mycobacterium tuberculosis: update 2015. Int J Tuberc Lung Dis 19(11):1276–1289
Lancini G (2014) In memory of Piero Sensi (1920–2013). J Antibiot 67(9):609–611
Herrera L, Jiménez S, Valverde A, Garcı́a-Aranda MA, Sáez-Nieto JA (2003) Molecular analysis of rifampicin-resistant Mycobacterium tuberculosis isolated in Spain (1996–2001). Description of new mutations in the rpoB gene and review of the literature. Int J Antimicrobial Agents 21(5):403–408
Kumar S, Jena L (2014) Understanding rifampicin resistance in tuberculosis through a computational approach. Genomics Inform 12(4):276
Pandey B, Grover S, Tyagi C, Goyal S, Jamal S, Singh A, Kaur J, Grover A (2016) Molecular principles behind pyrazinamide resistance due to mutations in panD gene in Mycobacterium tuberculosis. Gene 581(1):31–42
Njire M, Tan Y, Mugweru J, Wang C, Guo J, Yew W, Tan S, Zhang T (2016) Pyrazinamide resistance in Mycobacterium tuberculosis: review and update. Adv Med Sci 61(1):63–71
Nguyen L (2016) Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch Toxicol 90(7):1585–1604
Kanji A, Hasan R, Hasan Z (2019) Efflux pump as alternate mechanism for drug resistance in Mycobacterium tuberculosis. Indian J Tuberc 66(1):20–25
Hao P, Shi-Liang Z, Ju L, Ya-**n D, Biao H, Xu W, Min-Tao H, Shou-Gang K, Ke W (2011) The role of ABC efflux pump, Rv1456c-Rv1457c-Rv1458c, from Mycobacterium tuberculosis clinical isolates in China. Folia Microbiol 56(6):549–553
Danilchanka O, Mailaender C, Niederweis M (2008) Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob Agents Chemother 52(7):2503–2511
Zhang Y, Zhang J, Cui P, Zhang Y, Zhang W (2017) Identification of novel efflux proteins Rv0191, Rv3756c, Rv3008, and Rv1667c involved in pyrazinamide resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 61(8)
Duan W, Li X, Ge Y, Yu Z, Li P, Li J, Qin L, **e J (2019) Mycobacterium tuberculosis Rv1473 is a novel macrolides ABC efflux pump regulated by WhiB7. Future Microbiol 14(1):47–59
Choudhuri BS, Bhakta S, Barik R, Basu J, Kundu M, Chakrabarti P (2002) Overexpression and functional characterization of an ABC (ATP-binding cassette) transporter encoded by the genes drrA and drrB of Mycobacterium tuberculosis. Biochem J 367(1):279–285
Li P, Gu Y, Li J, **e L, Li X, **e J (2017) Mycobacterium tuberculosis major facilitator superfamily transporters. J Membr Biol 250(6):573–585
Gupta AK, Reddy VP, Lavania M, Chauhan D, Venkatesan K, Sharma V, Tyagi A, Katoch V (2010) jefA (Rv2459), a drug efflux gene in Mycobacterium tuberculosis confers resistance to isoniazid & ethambutol. Indian J Med Res 132(2):176–188
Gupta AK, Katoch VM, Chauhan DS, Sharma R, Singh M, Venkatesan K, Sharma VD (2010) Microarray analysis of efflux pump genes in multidrug-resistant Mycobacterium tuberculosis during stress induced by common anti-tuberculous drugs. Microb Drug Resist 16(1):21–28
Silva PE, Bigi F, de la Paz Santangelo M, Romano MI, Martı́n C, Cataldi A, Aı́nsa JA (2001) Characterization of P55, a multidrug efflux pump in Mycobacterium bovis and Mycobacterium tuberculosis. Antimicrob Agents Chemother 45(3):800–804
Ramón-García S, Martín C, Thompson CJ, Aínsa JA (2009) Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrob Agents Chemother 53(9):3675–3682
Cloete R, Kapp E, Joubert J, Christoffels A, Malan SF (2018) Molecular modelling and simulation studies of the Mycobacterium tuberculosis multidrug efflux pump protein Rv1258c. PLoS ONE 13(11):e0207605
Katoch VM (2019) Molecular basis of drug resistance in Mycobacteria. In: Pathogenicity and drug resistance of human pathogens. Springer, pp 3–31
Doran JL, Pang Y, Mdluli KE, Moran AJ, Victor TC, Stokes RW, Mahenthiralingam E, Kreiswirth BN, Butt JL, Baron GS (1997) Mycobacterium tuberculosis efpA encodes an efflux protein of the QacA transporter family. Clin Diagn Lab Immunol 4(1):23–32
Li X-Z, Zhang L, Nikaido H (2004) Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob Agents Chemother 48(7):2415–2423
Batt SM, Jabeen T, Bhowruth V, Quill L, Lund PA, Eggeling L, Alderwick LJ, Fütterer K, Besra GS (2012) Structural basis of inhibition of Mycobacterium tuberculosis DprE1 by benzothiazinone inhibitors. Proc Natl Acad Sci 109(28):11354–11359
Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marbán E (2013) Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 5(2):191–209
He L, Wang X, Cui P, ** J, Chen J, Zhang W, Zhang Y (2015) ubiA (Rv3806c) encoding DPPR synthase involved in cell wall synthesis is associated with ethambutol resistance in Mycobacterium tuberculosis. Tuberculosis 95(2):149–154
Ramón-García S, Martín C, De Rossi E, Aínsa JA (2007) Contribution of the Rv2333c efflux pump (the Stp protein) from Mycobacterium tuberculosis to intrinsic antibiotic resistance in Mycobacterium bovis BCG. J Antimicrob Chemother 59(3):544–547
Rodrigues L, Cravo P, Viveiros M (2020) Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: a new strategy to revisit mycobacterial targets and repurpose old drugs. Exp Rev Anti-Infect Ther, pp 1–17
Singh R, Dwivedi SP, Gaharwar US, Meena R, Rajamani P, Prasad T (2020) Recent updates on drug resistance in Mycobacterium tuberculosis. J Appl Microbiol 128(6):1547–1567
Te Brake LH, van den Heuvel JJ, Buaben AO, van Crevel R, Bilos A, Russel FG, Aarnoutse RE, Koenderink JB (2016) Moxifloxacin is a potent in vitro inhibitor of OCT-and MATE-mediated transport of metformin and ethambutol. Antimicrob Agents Chemother 60(12):7105–7114
Pasca MR, Guglierame P, De Rossi E, Zara F, Riccardi G (2005) mmpL7 gene of Mycobacterium tuberculosis is responsible for isoniazid efflux in Mycobacterium smegmatis. Antimicrob Agents Chemother 49(11):4775–4777
Briffotaux J, Huang W, Wang X, Gicquel B (2017) MmpS5/MmpL5 as an efflux pump in Mycobacterium species. Tuberculosis 107:13–19
Poulsen BE, Deber CM (2012) Drug efflux by a small multidrug resistance protein is inhibited by a transmembrane peptide. Antimicrob Agents Chemother 56(7):3911–3916
De Rossi E, Branzoni M, Cantoni R, Milano A, Riccardi G, Ciferri O (1998) mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J Bacteriol 180(22):6068–6071
Rodrigues L, Villellas C, Bailo R, Viveiros M, Aínsa JA (2013) Role of the Mmr efflux pump in drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 57(2):751–757
Hegde SS, Vetting MW, Roderick SL, Mitchenall LA, Maxwell A, Takiff HE, Blanchard JS (2005) A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 308(5727):1480–1483
Hameed PS, Raichurkar A, Madhavapeddi P, Menasinakai S, Sharma S, Kaur P, Nandishaiah R, Panduga V, Reddy J, Sambandamurthy VK (2014) Benzimidazoles: novel mycobacterial gyrase inhibitors from scaffold morphing. ACS Med Chem Lett 5(7):820–825
Flores AR, Parsons LM, Pavelka MS Jr (2005) Genetic analysis of the β-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to β-lactam antibiotics. Microbiology 151(2):521–532
Page MG (2012) Beta-lactam antibiotics. In: Antibiotic discovery and development. Springer, pp 79–117
Kashyap A, Singh PK, Silakari O (2018) Mechanistic investigation of resistance via drug-inactivating enzymes in Mycobacterium tuberculosis. Drug Metab Rev 50(4):448–465
Tremblay LW, Xu H, Blanchard JS: Structures of the Michaelis complex (1.2 Å) and the covalent acyl intermediate (2.0 Å) of cefamandole bound in the active sites of the Mycobacterium tuberculosis β-lactamase K73A and E166A mutants. Biochemistry 49(45):9685–9687
Upton A, Mushtaq A, Victor T, Sampson S, Sandy J, Smith DM, Van Helden P, Sim E (2001) Arylamine N-acetyltransferase of Mycobacterium tuberculosis is a polymorphic enzyme and a site of isoniazid metabolism. Mol Microbiol 42(2):309–317
Payton M, Auty R, Delgoda R, Everett M, Sim E (1999) Cloning and characterization of arylamine N-acetyltransferase genes from Mycobacterium smegmatis and Mycobacterium tuberculosis: increased expression results in isoniazid resistance. J Bacteriol 181(4):1343–1347
Payton M, Gifford C, Schartau P, Hagemeier C, Mushtaq A, Lucas S, Pinter K, Sim E (2001) Evidence towards the role of arylamine N-acetyltransferase in Mycobacterium smegmatis and development of a specific antiserum against the homologous enzyme of Mycobacterium tuberculosis. Microbiology 147(12):3295–3302
Sikora AL, Frankel BA, Blanchard JS (2008) Kinetic and chemical mechanism of arylamine N-acetyltransferase from Mycobacterium tuberculosis. Biochemistry 47(40):10781–10789
Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S (2011) Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc Natl Acad Sci 108(24):9804–9808
Gao C, Peng C, Shi Y, You X, Ran K, **ong L, Ye T-H, Zhang L, Wang N, Zhu Y (2016) Benzothiazinethione is a potent preclinical candidate for the treatment of drug-resistant tuberculosis. Sci Rep 6(1):1–9
Warrier T, Martinez-Hoyos M, Marin-Amieva M, Colmenarejo G, Porras-De Francisco E, Alvarez-Pedraglio AI, Fraile-Gabaldon MT, Torres-Gomez PA, Lopez-Quezada L, Gold B (2015) Identification of novel antimycobacterial compounds by screening a pharmaceutical small-molecule library against nonreplicating Mycobacterium tuberculosis. ACS Infect Dis 1(12):580–585
Warrier T, Kapilashrami K, Argyrou A, Ioerger TR, Little D, Murphy KC, Nandakumar M, Park S, Gold B, Mi J (2016) N-methylation of a bactericidal compound as a resistance mechanism in Mycobacterium tuberculosis. Proc Natl Acad Sci 113(31):E4523–E4530
Wermuth CG (2003) Analog design. In: Burger’s medicinal chemistry and drug discovery, pp 167–180
Vosátka R, Krátký M, Švarcová M, Janoušek J, Stolaříková J, Madacki J, Huszár S, Mikušová K, Korduláková J, Trejtnar F (2018) New lipophilic isoniazid derivatives and their 1,3,4-oxadiazole analogues: synthesis, antimycobacterial activity and investigation of their mechanism of action. Eur J Med Chem 151:824–835
Bhoi MN, Borad MA, Jethava DJ, Acharya PT, Pithawala EA, Patel CN, Pandya HA, Patel HD (2019) Synthesis, biological evaluation and computational study of novel isoniazid containing 4H-Pyrimido [2,1-b] benzothiazoles derivatives. Eur J Med Chem 177:12–31
De P, Koumba Yoya G, Constant P, Bedos-Belval F, Duran H, Saffon N, Daffé M, Baltas M (2011) Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J Med Chem 54(5):1449–1461
Kumar D, Khare G, Kidwai S, Tyagi AK, Singh R, Rawat DS (2014) Synthesis of novel 1,2,3-triazole derivatives of isoniazid and their in vitro and in vivo antimycobacterial activity evaluation. Eur J Med Chem 81:301–313
Tiwari R, Miller PA, Chiarelli LR, Mori G, Šarkan M, Centárová I, Cho S, Mikušová K, Franzblau SG, Oliver AG (2016) Design, syntheses, and anti-TB activity of 1,3-benzothiazinone azide and click chemistry products inspired by BTZ043. ACS Med Chem Lett 7(3):266–270
Hearn MJ, Cynamon MH (2004) Design and synthesis of antituberculars: preparation and evaluation against Mycobacterium tuberculosis of an isoniazid Schiff base. J Antimicrob Chemother 53(2):185–191
Shingnapurkar D, Dandawate P, Anson CE, Powell AK, Afrasiabi Z, Sinn E, Pandit S, Swamy KV, Franzblau S, Padhye S (2012) Synthesis and characterization of pyruvate–isoniazid analogs and their copper complexes as potential ICL inhibitors. Bioorg Med Chem Lett 22(9):3172–3176
Dandawate P, Vemuri K, Swamy KV, Khan EM, Sritharan M, Padhye S (2014) Synthesis, characterization, molecular docking and anti-tubercular activity of plumbagin-isoniazid analog and its β-cyclodextrin conjugate. Bioorg Med Chem Lett 24(21):5070–5075
Brooke EW, Davies SG, Mulvaney AW, Okada M, Pompeo F, Sim E, Vickers RJ, Westwood IM (2003) Synthesis and in vitro evaluation of novel small molecule inhibitors of bacterial arylamine N-acetyltransferases (NATs). Bioorg Med Chem Lett 13(15):2527–2530
Willby MJ, Green KD, Gajadeera CS, Hou C, Tsodikov OV, Posey JE, Garneau-Tsodikova S (2016) Potent inhibitors of acetyltransferase Eis overcome kanamycin resistance in Mycobacterium tuberculosis. ACS Chem Biol 11(6):1639–1646
Garzan A, Willby MJ, Ngo HX, Gajadeera CS, Green KD, Holbrook SY, Hou C, Posey JE, Tsodikov OV, Garneau-Tsodikova S (2017) Combating enhanced intracellular survival (Eis)-mediated kanamycin resistance of Mycobacterium tuberculosis by novel pyrrolo [1,5-a] pyrazine-based Eis inhibitors. ACS Infect Dis 3(4):302–309
Garzan A, Willby MJ, Green KD, Gajadeera CS, Hou C, Tsodikov OV, Posey JE, Garneau-Tsodikova S (2016) Sulfonamide-based inhibitors of aminoglycoside acetyltransferase Eis abolish resistance to kanamycin in Mycobacterium tuberculosis. J Med Chem 59(23):10619–10628
Ngo HX, Green KD, Gajadeera CS, Willby MJ, Holbrook SY, Hou C, Garzan A, Mayhoub AS, Posey JE, Tsodikov OV (2018) Potent 1,2,4-triazino [5,6b] indole-3-thioether inhibitors of the kanamycin resistance enzyme Eis from Mycobacterium tuberculosis. ACS Infect Dis 4(6):1030–1040
Garzan A, Willby MJ, Green KD, Tsodikov OV, Posey JE, Garneau-Tsodikova S (2016) Discovery and optimization of two Eis inhibitor families as kanamycin adjuvants against drug-resistant M. tuberculosis. ACS Med Chem Lett 7(12):1219–1221
Kurz SG, Hazra S, Bethel CR, Romagnoli C, Caselli E, Prati F, Blanchard JS, Bonomo RA (2015) Inhibiting the β-lactamase of Mycobacterium tuberculosis (M. tb) with novel boronic acid transition-state inhibitors (BATSIs). ACS Infect Dis 1(6):234–242
Hazra S, Kurz SG, Wolff K, Nguyen L, Bonomo RA, Blanchard JS (2015) Kinetic and structural characterization of the interaction of 6-methylidene penem 2 with the β-lactamase from Mycobacterium tuberculosis. Biochemistry 54(36):5657–5664
Iannazzo L, Soroka D, Triboulet S, Fonvielle M, Compain F, Dubée V, Mainardi J-L, Hugonnet J-E, Braud E, Arthur M (2016) Routes of synthesis of carbapenems for optimizing both the inactivation of l,d-transpeptidase LdtMt1 of Mycobacterium tuberculosis and the stability toward hydrolysis by β-lactamase BlaC. J Med Chem 59(7):3427–3438
Xu H, Hazra S, Blanchard JS (2012) NXL104 irreversibly inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry 51(22):4551–4557
Caminero JA, Piubello A, Scardigli A, Migliori GB (2017) Proposal for a standardised treatment regimen to manage pre-and extensively drug-resistant tuberculosis cases. Eur Respir Soc
Tiberi S, Scardigli A, Centis R, D’Ambrosio L, Munoz-Torrico M, Salazar-Lezama MA, Spanevello A, Visca D, Zumla A, Migliori GB (2017) Classifying new anti-tuberculosis drugs: rationale and future perspectives. Int J Infect Dis 56:181–184
Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, Jaramillo E, Weyer K (2017) World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 49(3)
World Health Organization (2016) WHO treatment guidelines for drug-resistant tuberculosis. World Health Organization
Caminero JA, Scardigli A (2015) Classification of antituberculosis drugs: a new proposal based on the most recent evidence. Eur Respir Soc
Abbate E, Vescovo M, Natiello M, Cufré M, García A, Ambroggi M, Poggi S, Símboli N, Ritacco V (2007) Tuberculosis extensamente resistente (XDR-TB) en Argentina: aspectos destacables epidemiológicos, bacteriológicos, terapéuticos y evolutivo
Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, Van Soolingen D (2010) Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! Int J Antimicrob Agents 35(6):524–526
Gopal M, Padayatchi N, Metcalfe J, O’Donnell M (2013) Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 17(8):1001–1007
Chhabra N, Aseri M, Dixit R, Gaur S (2012) Pharmacotherapy for multidrug resistant tuberculosis. J Pharmacol Pharmacother 3(2):98
Zhang T, Jiang G, Shu’an Wen FH, Wang F, Huang H, Pang Y (2019) Para-aminosalicylic acid increases the susceptibility to isoniazid in clinical isolates of Mycobacterium tuberculosis. Infect Drug Resist 12:825
Fan Y-L, Wu J-B, Cheng X-W, Zhang F-Z, Feng L-S (2018) Fluoroquinolone derivatives and their anti-tubercular activities. Europ J Med Chem 146:554–563
Kumar B, Sharma D, Sharma P, Katoch VM, Venkatesan K, Bisht D (2013) Proteomic analysis of Mycobacterium tuberculosis isolates resistant to kanamycin and amikacin. J Proteomics 94:68–77
Rozwarski DA, Grant GA, Barton DH, Jacobs WR, Sacchettini JC (1998) Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279(5347):98–102
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Verma, H., Choudhary, S., Silakari, O. (2023). Resistance in Tuberculosis: Molecular Mechanisms and Modulation. In: Rezaei, N. (eds) Tuberculosis. Integrated Science, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-031-15955-8_19
Download citation
DOI: https://doi.org/10.1007/978-3-031-15955-8_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15954-1
Online ISBN: 978-3-031-15955-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)