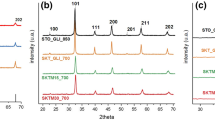
Abstract
Strontium stannate (SrSnO3) is a perovskite, which has been extensively studied due to its potential technological applications. In this work, SrSnO3 was doped with transition metals (Cu, Fe, Ni) by the modified Pechini method and evaluated in the catalytic reduction of NO with CO. The perovskite structure was obtained as the major phase for all samples. The catalytic activity of SrSnO3 was highly improved by all the dopants, as only 10% of conversion was obtained for SrSnO3, whereas samples with 5% of Cu presented the highest conversions, reaching 100% of NO into N2 and 100% of CO into CO2 at 550 °C. Conversions of 85% of NO into N2 and 90% of CO into CO2 were obtained for Ni doped samples at 600 °C, while 62% of conversion was obtained for Fe-doped samples, for both reactions. These results indicate that Cu is the best dopant, which leads to the greatest NO conversion with a smaller amount of dopant.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Song, J., Wang, Z., Cheng, X., Wang, X.: State-of-art review of NO reduction technologies by CO, CH4 and H2. Process 9, 563(1–28) (2021). https://doi.org/10.3390/pr9030563
Han, L., Cai, S., Gao, M., Hasegawa, J., Wang, P., Zhang, J., Shi, L., Zhang, D.: Selective catalytic reduction of NOx with NH3 by using novel catalysts: state of the art and future prospects. Chem. Rev. 119(19), 10916–10976 (2019). https://doi.org/10.1021/acs.chemrev.9b00202
Erickson, L.M., Newwark, G., Higgins, M.J., Wang, Z.: Nitrogen oxides and ozone in urban air: a review of 50 plusyears of progress. Environ. Progr. Sustain. Energy 39(e13484), 1–9 (2020). https://doi.org/10.1002/ep.13484
Lin, Y., Cao, Y., Yao, Q., Chai, O.J.H., **e, J.: Engineering noble metal nanomaterials for pollutant decomposition. Ind. Eng. Chem. Res. 59, 20561–20581 (2020). https://doi.org/10.1021/acs.iecr.0c04258
Liu, C., Kubota, H., Amanda, T., Toyao, T., Maeno, Z., Ogura, M., Nakazawa, N., Inagaki, S., Kubota, Y., Shimizu, K.: Selective catalytic reduction of NO over Cu-AFX zeolites: mechanistic insights from in situ/operando spectroscopic and DFT studies. Catal. Sci. Technol. 11, 4459–4470 (2021). https://doi.org/10.1039/D1CY00282A
Shen, Q., Dong, S., Li, S., Yang, G., Pan, X.: A review on the catalytic decomposition of NO by perovskite-type oxides. Catalyts 11, 622(1–12) (2021). https://doi.org/10.3390/catal11050622
Zhang, R., Villanueva, A., Alamdari, H., Kaliaguine, S.: Cu and Pd-substituted nanoscale Fe-based perovskites for selective catalytic reduction of NO by propene. J. Catal. 237(2), 368–380 (2006). https://doi.org/10.1016/j.jcat.2005.11.019
Hodjati, S., Vaezzadeh, K., Petit, C., Pitchon, V., Kiennemann, A.: Absorption/desorption of NOx process on perovskites: performances to remove NOx from a lean exhaust gas. Appl. Catal. B 26(1), 5–16 (2000). https://doi.org/10.1016/S0926-3373(99)00143-5
Smith, J.M., Ness, H.C.V., Abbott, M.M.: Introdução à Termodinâmica da Engenharia Química. ed. 7, Rio de Janeiro: LTC (2007)
Deng, C., Huang, Q., Zhu, X., Hu, Q., Su, W., Qian, J., Dong, L., Li, B., Fan, M., Liang, C.: The influence of Mn-doped CeO2 on the activity of CuO/CeO2 in CO oxidation and NO + CO model reaction. Appl. Surf. Sci. 389, 1033–1049 (2016). https://doi.org/10.1016/j.apsusc.2016.08.035
Tanabe, E.Y., Assaf, E.E.: Óxidos do tipo perovskita para reação de NO com CO. Quim. Nova 32(5), 1129–1133 (2009). https://doi.org/10.1590/S0100-40422009000500009
Castillo, S., Pineda, M.M., Gómez, R.: Reduction of NO by CO under oxidizing conditions over Pt and Rh supported on Al2O3-ZrO2 binary oxides. Catal. Commun. 2(10), 295–300 (2001). https://doi.org/10.1016/s1566-7367(01)00049-8
Leontiou, A.A., Ladavos, A.K., Pomonis, P.J.: Catalytic NO reduction with CO on La1−xSrx(Fe3+/Fe4+)O3± δ perovskite-type mixed oxides (x = 0.00, 0.15, 0.30, 0.40, 0.60, 0.70, 0.80, and 0.90). Appl. Catal. A 241(1–2), 133–141 (2003). https://doi.org/10.1016/S0926-860X(02)00457-X
Zhang, R., Villanueva, A., Alamdari, H., Kaliaguine, S.: Reduction of NO by CO over nanoscale LaCo1−xCuxO3 and LaMn1−xCuxO3 perovskites. J. Mol. Catal. A: Chem. 258(1–2), 22–34 (2006). https://doi.org/10.1016/j.molcata.2006.05.008
Teraoka, Y., Nii, H., Kagawa, S., Jansson, K., Nygren, M.: Influence of the simultaneous substitution of Cu and Ru in the perovskite-type (La, Sr)MO3 (M=Al, Mn, Fe, Co) on the catalytic activity for CO oxidation and CO–NO reactions. Appl. Catal. A 194–195, 35–41 (2000). https://doi.org/10.1016/S0926-860X(99)00351-8
Hadjarab, B., Bouguelia, A., Trati, M.: Synthesis, physical and photo electrochemical characterization of La-doped SrSnO3. J. Phys. Chem. Solids 68(8), 1491–1499 (2007). https://doi.org/10.1016/j.jpcs.2007.03.013
Azad, A.M., Hon, N.C.: Characterization of BaSnO3-based ceramics: Part 1. Synthesis, processing and microstructural development. J. Alloy. Compd. 270(1–2), 95–106 (1998). https://doi.org/10.1016/S0925-8388(98)00370-3
Honório, L.M.C., Santos, M.V.B., Filho, E.C.S., Osajima, J.A., Maia, A.S., Santos, I.M.G.: Alkaline earth stannates applied in photocatalysis: prospection and review of literature. Cerâmica 64, 559–569 (2018). https://doi.org/10.1590/0366-69132018643722480
Zidi, N., Omeiri, S., Hadjarab, B., Bouguelia, A., Akroun, A., Trari, M.: Transport properties and photoelectrochemical characterization of oxygen-deficient ASnO3 (A= Ca, Sr and Ba). Physica B 405(16), 3355–3359 (2010). https://doi.org/10.1016/j.physb.2010.05.004
Mizoguchi, H., Eng, H.W., Woodward, P.M.: Probing the electronic structures of ternary perovskite and pyrochlore oxides containing Sn4++ or Sb5+. Inorg. Chem. 43(5), 1667–1680 (2004). https://doi.org/10.1021/ic034551c
Alves, M.C.F., Souza, S.C., Silva, M.R.S., Paris, E.C., Lima, S.J.G., Gomes, R.M., Longo, E., Souza, A.G., Santos, I.M.G.: Thermal analysis applied in the crystallization study of SrSnO3. J. Therm. Anal. Calorim. 97, 179–183 (2009). https://doi.org/10.1007/s10973-009-0242-x
Bohnemann, J., Libanori, R., Moreira, M.L., Longo, E.: High-efficient microwave synthesis and characterisation of SrSnO3. Chem. Eng. J. 155(3), 905–909 (2009). https://doi.org/10.1016/j.cej.2009.09.004
Peña, M.A., Fierro, J.L.: Chemical structures and performance of perovskite oxides. Chem. Rev. 101(7), 1981–2017 (2001). https://doi.org/10.1021/cr980129f
Song, S., Xu, L., He, Z., Ying, H., Chen, J., **ao, X., Yan, B.: Photocatalytic degradation of C.I. Direct Red 23 in aqueous solutions under UV irradiation using SrTiO3/CeO2 composite as the catalyst. J. Hazard. Mater. 152(3), 1301−1308 (2008). https://doi.org/10.1016/j.jhazmat.2007.08.004
Ferri, D., Forni, L.: Methane combustion on some perovskite-like mixed oxides. Appl. Catal. B 16(2), 119–126 (1998). https://doi.org/10.1016/S0926-3373(97)00065-9
Zhu, J., Thomas, A.: Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B 92(3–4), 225–233 (2009). https://doi.org/10.1016/j.apcatb.2009.08.008
Chen, L., Si, Z., Wu, X., Weng, D.: DRIFT study of CuO–CeO–TiO2 mixed oxides for NOx reduction with NH3 at low temperatures. ACS Appl. Mater. Interf. 6(6), 8134–8145 (2014). https://doi.org/10.1021/am5004969
Tien-Thao, N., Alamdari, H., Kaliaguine, S.: Characterization and reactivity of nanoscale La(Co, Cu)O3 perovskite catalyst precursors for CO hydrogenation. J. Solid State Chem. 181(8), 2006–2019 (2008). https://doi.org/10.1016/j.jssc.2007.11.016
Zhu, J., Zhao, Z., **ao, D., Li, J., Yang, X., Wu, Y.: Study of La2−xSrxCuO4 (x = 0.0, 0.5, 1.0) catalysts for NO+CO reaction from the measurements of O2-TPD, H2–TPR and cyclic voltammetry. J. Mol. Catal. A: Chem. 238(1–2), 35–40 (2005). https://doi.org/10.1016/j.molcata.2005.03.036
Giannakas, A.E., Leontiou, A.A., Ladavos, A.K., Pomonis, P.J.: Characterization and catalytic investigation of NO + CO reaction on perovskites of the general formula LaxM1-xFeO3 (M = Sr and/or Ce) prepared via a reverse micelles microemulsion route. Appl. Catal. A 309(2), 254–262 (2006). https://doi.org/10.1016/j.apcata.2006.05.016
Zhang, R., Alamdari, H., Kaliaguine, S.: Fe-based perovskites substituted by copper and palladium for NO+ CO reaction. J. Catal. 242(2), 241–253 (2006). https://doi.org/10.1016/j.jcat.2006.05.033
He, H., Liu, M., Dai, H., Qiu, W., Zi, X.: An investigation of NO/CO reaction over perovskite-type oxide La0.8Ce0.2B0.4Mn0.6O3 (B = Cu or Ag) catalysts synthesized by reverse microemulsion. Catal. Today 126(3–4), 290–295 (2007). https://doi.org/10.1016/j.cattod.2007.06.004
Dai, H., He, H., Li, P., Gao, L., Au, C.H.: The relationship of structural defect–redox property–catalytic performance of perovskites and their related compounds for CO and NOx removal. Catal. Today 90(3–4), 231–244 (2004). https://doi.org/10.1016/j.cattod.2004.04.031
Wu, Y., Li, L., Chu, B., Yi, Y., Qin, Z., Fan, M., Qin, Q., He, H., Zhang, L., Dong, L., Li, B., Dong, L., Li, B., Dong, L.: Catalytic reduction of NO by CO over B-site partially substituted LaM0.25Co0.75O3 (M = Cu, Mn, Fe) perovskite oxide catalysts: the correlation between physicochemical properties and catalytic performance. Appl. Catal. A 568, 43–53 (2018). https://doi.org/10.1016/j.apcata.2018.09.022
Wood, D.L., Tauc, J.: Weak absorption tails in amorphous semiconductors. Phys. Rev. B 5(8), 3144–3151 (1972). https://doi.org/10.1103/PhysRevB
Nakamoto, K.: Infrared and Raman Spectra of Inorganic and Coordination Compounds. John Wiley & Sons, New York, Inc (1986)
Nyquist, R.A., Kagel, R.O.: Infrared Spectra Inorganic Compounds. Academic Press, New York, Inc (1971)
Moreira, E., Henriques, J.M., Azevedo, D.L., Caetano, E.W.S., Freire, V.N., Albuquerque, E.L.: Structural, optoelectronic, infrared and Raman spectra of orthorhombic SrSnO3 from DFTcalculations. J. Solid State Chem. 184(4), 921–928 (2011). https://doi.org/10.1016/j.jssc.2011.02.009
Last, J.T.: Infrared-absorption studies on barium titanate and related materials. Phys. Rev. 105(6), 1740–1750 (1956). https://doi.org/10.1103/PhysRev.105.1740
Pfaff, G., Hildenbrand, V.D., Fuess, H.: Spectroscopic study of amorphous precursors for alkaline-earth titanates and stannates. J. Mater. Sci. Lett. 17(23), 1983–1985 (1998). https://doi.org/10.1023/A:1006652405086
Azad, A.-M., Shyan, L.L.W., Yen, P.T.: Synthesis, processing and microstructural characterization of CaSnO3 and SrSnO3 ceramics. J. Alloy. Compd. 282(1–2), 109–124 (1999). https://doi.org/10.1016/S0925-8388(98)00808-1
Chiang, Y.M., Bimie III, D.P., Kingery, W.D.: Physical Ceramics. John Wiley & Sons, New York (1997)
Tarrida, M., Larguem, H., Madon, M.: Structural investigations of (Ca, Sr)ZrO3 and Ca(Sn, Zr)O3 perovskite compounds. Phys. Chem. Miner. 36, 403–413 (2009). https://doi.org/10.1007/s00269-008-0286-7
Doroftei, C., Popa, P.D., Iacomi, F.: Study of the influence of nickel ions substitutes in barium stannates used as humidity resistive sensors. Sens. Actuators, A 173(1), 24–29 (2012). https://doi.org/10.1016/j.sna.2011.10.007
Kaabar, W., Botta, S., Devonshire, R.: Raman spectroscopic study of mixed carbonate materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 78(1), 136–141 (2011). https://doi.org/10.1016/j.saa.2010.09.011
Liu, Z.C., Chen, H.R., Huang, W.M., Gu, J.L., Bu, W.B., Hua, Z.L., Shi, J.L.: Synthesis of a new SnO2/mesoporous silica composite with room-temperature photoluminescence. Microporous Mesoporous Mater. 89, 270–275 (2006). https://doi.org/10.1016/j.micromeso.2005.10.037
Rao, L.S., Reddy, M.S., Rao, D.K., Veeraiah, N.: Influence of redox behavior of copper ions on dielectric and spectroscopic properties of Li2O–MoO3–B2O3:CuO glass system. Solid State Sci. 11, 578–587 (2009). https://doi.org/10.1016/j.solidstatesciences.2008.06.022
Sorlí, S.M.A., Tena, J.A., Badenes, J.C., Calbo, M., Llusar, G.M., Monrós, G.: Structure and color of NixA1–3xB2xO2 (A=Ti, Sn; B=Sb, Nb) solid solutions. J. Eur. Ceram. Soc. 24(8), 2425–2432 (2004). https://doi.org/10.1016/j.jeurceramsoc.2003.07.012
Dondi, M., Cruciani, G., Guarini, G., Matteucci, F., Raimondo, M.: The role of counterions (Mo, Nb, Sb, W) in Cr-, Mn-, Ni- and V-doped rutile ceramic pigments: Part 2. Colour and technological properties. Ceram. Int. 32(4), 393–405 (2006). https://doi.org/10.1016/j.ceramint.2005.03.015
Reddy, K.N., Reddy, G.S., Reddy, S.L., Rao, P.S.: Optical absorption and EPR spectral studies of vauquelinite. Cryst. Res. Technol. 41(8), 818–821 (2006). https://doi.org/10.1080/10420150008211834
Lee, J.D.: Concise Inorganic Chemistry, 5th edn. Chapman & Hall, London, Inc (1996)
Rajyasree, Ch., Rao, D.K.: Spectroscopic investigations on alkali earth bismuth borate glasses doped with CuO. J. Non-Cryst. Solids 357(3), 836–841 (2011). https://doi.org/10.1016/j.jnoncrysol.2010.11.008
Liu, Q., Dai, J., Zhang, X., Zhu, G., Liu, Z., Ding, G.: Perovskite-type transparent and conductive oxide films: Sb- and Nd-doped SrSnO3. Thin Solid Films 519(18), 6059–6063 (2011). https://doi.org/10.1016/j.tsf.2011.03.038
Fritz, A., Pitchon, V.: The current state of research on automotive lean NOx catalysis. Appl. Catal. B 113(1), 1–5 (1997). https://doi.org/10.1016/S0926-3373(96)00102-6
Lin, C.-H., Liu, L.-G.: Post-aragonite phase transitions in strontianite and cerussite—A high-pressure raman spectroscopy study. J. Phys. Chem. Solids 58(6), 977–987 (1997). https://doi.org/10.1016/S0022-3697(96)00201-6
House, J.E.: Inorganic Chemistry. Academic Press, Canada (2008)
Rao, J.L., Murali, A., Rao, E.D.: Electron paramagnetic resonance and optical absorption spectra of Fe(III) ions in alkali zinc boro sulphate glasses. J. Non-Cryst. Solids 202(3), 215–221 (1996). https://doi.org/10.1016/0022-3093(96)00199-8
Taran, M.N., Langer, K.: Electronic absorption spectra of Fe3+ in andradite and epidote at different temperatures and pressures. Eur. J. Miner. 12(1), 7–15 (2000). https://doi.org/10.1127/0935-1221/2000/0012-0007
Dazhi, W., Shulin, W., Jun, C., Suyuan, Z., Fangqing, L.: Microstructure of SnO2. Phys. Rev. B 49(20), 14282–14285 (1994). https://doi.org/10.1103/PhysRevB.49.14282
Mesíková, Z., Sulcová, P., Trojan, M.: Synthesis and description of SrSn0.6In0.4O3 perovskite pigments. J. Therm. Anal. Calorim. 91(1), 163–166 (2008). https://doi.org/10.1007/s10973-007-8312-4
Zidi, N., Omeiri, S., Hadjarab, B., Bouguelia, A., Akroun, A., Trari, M.: Transport properties and photoelectrochemical characterization of oxygen-deficient ASnO3-δ (A= Ca, Sr and Ba). Physica B 405(16), 3355–3359 (2010). https://doi.org/10.1016/j.physb.2010.05.004
Lima, R.K.C., Batista, M.S., Wallau, M., Sanches, E.A., Mascarenhas, Y.P., Urquieta-González, E.A.: High specific surface area LaFeCo perovskites—Synthesis by nanocasting and catalytic behavior in the reduction of NO with CO. Appl. Catal. B 90(3–4), 441–450 (2009). https://doi.org/10.1016/j.apcatb.2009.04.004
Acknowledgements
The authors acknowledge Petrobras (Process Number 2014/00327-2), for the financing of this project and CNPq Proc. 151055/2012-2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lucena, G.L. et al. (2022). SrSnO3 Applied in the Reduction of NO by CO: Influence of Transition Metal Do** on the Catalytic Activity. In: Taft, C.A., de Lazaro, S.R. (eds) Research Topics in Bioactivity, Environment and Energy. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-07622-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-07622-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07621-3
Online ISBN: 978-3-031-07622-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)