Abstract
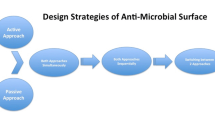
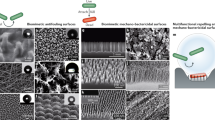
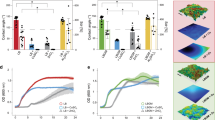
Biofilms can be understood as multilevel structures of microbial cells on a surface enclosed by an extracellular matrix. This type of organization is considered an adaptation mechanism that is adopted by many microorganisms to promote, among other things, protection against the exterior environment. This protective profile became a substantial problem in the case of bacterial infections since conventional treatment with antibiotics, for example, is less or noneffective. Thus, considering that bacterial cells need to attach to a surface to begin biofilm formation, some techniques have targeted the bacterial adhesion stage. These approaches are based on building materials with nanoengineered surfaces built with microdomain and nanodomain to minimize the contact with bacterial surface area and, therefore, promote interactions that keep cells from attaching to the surface.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81:7–11. https://doi.org/10.1016/j.jcma.2017.07.012.
Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7:1–7. https://doi.org/10.1038/ijos.2014.65.
Kumar P, Mishra S, Singh S. Advanced acuity in microbial biofilm genesis, development, associated clinical infections and control. Journal des Anti-infectieux. 2017;19:20–31. https://doi.org/10.1016/j.antinf.2017.01.002.
Khatoon Z, McTiernan CD, Suuronen EJ, Mah T-F, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067.
Stoica P, Chifiriuc MC, Rapa M, Lazăr V. Overview of biofilm-related problems in medical devices. In: Biofilms and implantable medical devices. Amsterdam: Elsevier; 2017. p. 3–23.
Koseki H, Yonekura A, Shida T, Yoda I, Horiuchi H, Morinaga Y, Yanagihara K, Sakoda H, Osaki M, Tomita M. Early staphylococcal biofilm formation on solid Orthopaedic implant materials: in vitro study. PLoS One. 2014;9:e107588. https://doi.org/10.1371/journal.pone.0107588.
Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P. Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Arch Microbiol. 2016;198:1–15. https://doi.org/10.1007/s00203-015-1148-6.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. https://doi.org/10.1038/nrmicro821.
Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the Dawn of the Postantibiotic era. Cold Spring Harb Perspect Med. 2013;3:a010306. https://doi.org/10.1101/cshperspect.a010306.
Ahmed MN, Porse A, Sommer MOA, Høiby N, Ciofu O. Evolution of antibiotic resistance in biofilm and planktonic Pseudomonas aeruginosa populations exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 2018;62. https://doi.org/10.1128/AAC.00320-18.
Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397–409. https://doi.org/10.1038/s41579-018-0019-y.
Chen L, Wen Y. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3:66–73. https://doi.org/10.4248/IJOS11022.
Römling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Åkerlund B. Microbial biofilm formation: a need to act. J Intern Med. 2014;276:98–110. https://doi.org/10.1111/joim.12242.
Tolker-Nielsen T. Biofilm development. Microbiol Spectr. 2015;3. https://doi.org/10.1128/microbiolspec.MB-0001-2014.
Birošová L, Mackuľak T, Bodík I, Ryba J, Škubák J, Grabic R. Pilot study of seasonal occurrence and distribution of antibiotics and drug resistant bacteria in wastewater treatment plants in Slovakia. Sci Total Environ. 2014;490:440–4. https://doi.org/10.1016/j.scitotenv.2014.05.030.
Alexander J, Bollmann A, Seitz W, Schwartz T. Microbiological characterization of aquatic microbiomes targeting taxonomical marker genes and antibiotic resistance genes of opportunistic bacteria. Sci Total Environ. 2015;512–513:316–25. https://doi.org/10.1016/j.scitotenv.2015.01.046.
Harris S, Morris C, Morris D, Cormican M, Cummins E. Antimicrobial resistant Escherichia coli in the municipal wastewater system: effect of hospital effluent and environmental fate. Sci Total Environ. 2014;468–469:1078–85. https://doi.org/10.1016/j.scitotenv.2013.09.017.
Tao C-W, Hsu B-M, Ji W-T, Hsu T-K, Kao P-M, Hsu C-P, Shen S-M, Shen T-Y, Wan T-J, Huang Y-L. Evaluation of five antibiotic resistance genes in wastewater treatment systems of swine farms by real-time PCR. Sci Total Environ. 2014;496:116–21. https://doi.org/10.1016/j.scitotenv.2014.07.024.
Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277–83.
Pogodin S, Hasan J, Baulin VA, Webb HK, Truong VK, Phong Nguyen TH, Boshkovikj V, Fluke CJ, Watson GS, Watson JA, Crawford RJ, Ivanova EP. Biophysical model of bacterial cell interactions with Nanopatterned Cicada wing surfaces. Biophys J. 2013;104:835–40. https://doi.org/10.1016/j.bpj.2012.12.046.
Lepore E, Giorcelli M, Saggese C, Tagliaferro A, Pugno N. Mimicking water striders’ legs superhydrophobicity and buoyancy with cabbage leaves and nanotube carpets. J Mater Res. 2013;28:976–83. https://doi.org/10.1557/jmr.2012.382.
Ensikat HJ, Ditsche-Kuru P, Neinhuis C, Barthlott W. Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J Nanotechnol. 2011;2:152–61. https://doi.org/10.3762/bjnano.2.19.
Ivanova EP, Hasan J, Webb HK, Truong VK, Watson GS, Watson JA, Baulin VA, Pogodin S, Wang JY, Tobin MJ, Löbbe C, Crawford RJ. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by Cicada wings. Small. 2012;8:2489–94. https://doi.org/10.1002/smll.201200528.
Lee W, Park S-J. Porous anodic aluminum oxide: anodization and templated synthesis of functional nanostructures. Chem Rev. 2014;114:7487–556. https://doi.org/10.1021/cr500002z.
Ferreira MGS, Zheludkevich ML, Tedim J, Yasakau KA. Self-healing nanocoatings for corrosion control. In: Corrosion protection and control using nanomaterials. Elsevier Cambridge; 2012. p. 213–63.
Wu Y, Zhao W, Wang W, Wang L, Xue Q. Novel anodic oxide film with self-sealing layer showing excellent corrosion resistance. Sci Rep. 2017;7. https://doi.org/10.1038/s41598-017-01549-y.
Buff H. Ueber das electrische Verhalten des Aluminiums. Annalen der Chemie und Pharmacie. 1857;102:265–84. https://doi.org/10.1002/jlac.18571020302.
Ali HO. Review of porous anodic aluminium oxide (AAO) applications for sensors, MEMS and biomedical devices. Trans IMF. 2017;95:290–6. https://doi.org/10.1080/00202967.2017.1358514.
Tsai B-F, Chen Y-C, Ou S-F, Wang K-K, Hsu Y-C. Fabrication of superhydrophobic titanium surfaces by anodization and surface mechanical attrition treatment. Int J Appl Ceram Technol. 2019;16:211–20. https://doi.org/10.1111/ijac.13104.
İzmir M, Ercan B. Anodization of titanium alloys for orthopedic applications. Front Chem Sci Eng. 2019;13:28–45. https://doi.org/10.1007/s11705-018-1759-y.
Ahmad A, Haq EU, Akhtar W, Arshad M, Ahmad Z. Synthesis and characterization of titania nanotubes by anodizing of titanium in fluoride containing electrolytes. Appl Nanosci. 2017;7:701–10. https://doi.org/10.1007/s13204-017-0608-5.
Omidvar H, Goodarzi S, Seif A, Azadmehr AR. Influence of anodization parameters on the morphology of TiO2 nanotube arrays. Superlattice Microst. 2011;50:26–39. https://doi.org/10.1016/j.spmi.2011.04.006.
Zhang Y, Lin T. Influence of duty cycle on properties of the superhydrophobic coating on an anodized magnesium alloy fabricated by pulse electrodeposition. Colloids Surf A Physicochem Eng Asp. 2019;568:43–50. https://doi.org/10.1016/j.colsurfa.2019.01.078.
Cipriano AF, Lin J, Miller C, Lin A, Cortez Alcaraz MC, Soria P, Liu H. Anodization of magnesium for biomedical applications – processing, characterization, degradation and cytocompatibility. Acta Biomater. 2017;62:397–417. https://doi.org/10.1016/j.actbio.2017.08.017.
Xue D, Yun Y, Schulz MJ, Shanov V. Corrosion protection of biodegradable magnesium implants using anodization. Mater Sci Eng C. 2011;31:215–23. https://doi.org/10.1016/j.msec.2010.08.019.
Kim S, Hyun S, Lee J, Lee KS, Lee W, Kim JK. Anodized aluminum oxide/polydimethylsiloxane hybrid Mold for roll-to-roll nanoimprinting. Adv Funct Mater. 2018;28:1800197. https://doi.org/10.1002/adfm.201800197.
Mymrin V, Molinetti A, Alekseev K, Avanci MA, Klitzke W, Silva DA, Ferraz FA, Iarozinski NA, Catai RE. Characterization of construction materials on the base of mortar waste, activated by aluminum anodization sludge and lime production waste. Constr Build Mater. 2019;212:202–9. https://doi.org/10.1016/j.conbuildmat.2019.03.276.
Yanagishita T, Murakoshi K, Kondo T, Masuda H. Preparation of superhydrophobic surfaces with micro/nano alumina molds. RSC Adv. 2018;8:36697–704. https://doi.org/10.1039/C8RA07497F.
Leontiev AP, Roslyakov IV, Napolskii KS. Complex influence of temperature on oxalic acid anodizing of aluminium. Electrochim Acta. 2019;319:88–94. https://doi.org/10.1016/j.electacta.2019.06.111.
Kozhukhova AE, du Preez SP, Bessarabov DG. Preparation of anodized aluminium oxide at high temperatures using low purity aluminium (Al6082). Surf Coat Technol. 2019;378:124970. https://doi.org/10.1016/j.surfcoat.2019.124970.
Hizal F, Rungraeng N, Lee J, Jun S, Busscher HJ, van der Mei HC, Choi C-H. Nanoengineered Superhydrophobic surfaces of aluminum with extremely low bacterial Adhesivity. ACS Appl Mater Interfaces. 2017;9:12118–29. https://doi.org/10.1021/acsami.7b01322.
Huang Q, Wu H, Cai P, Fein JB, Chen W. Atomic force microscopy measurements of bacterial adhesion and biofilm formation onto clay-sized particles. Sci Rep. 2015; 5. https://doi.org/10.1038/srep16857.
Li X, Logan BE. Analysis of bacterial adhesion using a gradient force analysis method and colloid probe atomic force microscopy. Langmuir. 2004;20:8817–22. https://doi.org/10.1021/la0488203.
Potthoff E, Ossola D, Zambelli T, Vorholt JA. Bacterial adhesion force quantification by fluidic force microscopy. Nanoscale. 2015;7:4070–9. https://doi.org/10.1039/C4NR06495J.
Hubenthal F. Noble metal nanoparticles: synthesis and optical properties. In: Comprehensive nanoscience and technology. London: Elsevier; 2011. p. 375–435.
Kim M, Ha D, Kim T. Cracking-assisted photolithography for mixed-scale patterning and nanofluidic applications. Nat Commun. 2015;6. https://doi.org/10.1038/ncomms7247.
Koek BH, Chisholm TV, Run AJ, Romijn J, Davey JP. An electron beam lithography tool with a Schottky emitter for wide range applications. Microelectron Eng. 1994;23:81–4. https://doi.org/10.1016/0167-9317(94)90109-0.
Moradian S, Modarres-Zadeh MJ, Abdolvand R. Thermal conductivity in nanoscale polysilicon structures with applications in sensors. Sensors Actuators A Phys. 2019;295:596–603. https://doi.org/10.1016/j.sna.2019.06.006.
Li X, Matino L, Zhang W, Klausen L, McGuire AF, Lubrano C, Zhao W, Santoro F, Cui B. A nanostructure platform for live-cell manipulation of membrane curvature. Nat Protoc. 2019;14:1772–802. https://doi.org/10.1038/s41596-019-0161-7.
Wu S, Zuber F, Maniura-Weber K, Brugger J, Ren Q. Nanostructured surface topographies have an effect on bactericidal activity. J Nanobiotechnol. 2018;16. https://doi.org/10.1186/s12951-018-0347-0.
Lutey AHA, Gemini L, Romoli L, Lazzini G, Fuso F, Faucon M, Kling R. Towards laser-textured antibacterial surfaces. Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-28454-2.
Ellinas K, Kefallinou D, Stamatakis K, Gogolides E, Tserepi A. Is there a threshold in the antibacterial action of Superhydrophobic surfaces? ACS Appl Mater Interfaces. 2017;9:39781–9. https://doi.org/10.1021/acsami.7b11402.
Ren T, Yang M, Wang K, Zhang Y, He J. CuO nanoparticles-containing highly transparent and Superhydrophobic coatings with extremely low bacterial adhesion and excellent bactericidal property. ACS Appl Mater Interfaces. 2018;10:25717–25. https://doi.org/10.1021/acsami.8b09945.
Acknowledgments
This work was partially financed by FAPERGS (Project # 19/2551-0001869-0).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
dos Santos da Silva, A., dos Santos, J.H.Z. (2021). Nanoengineered Surfaces as a Tool Against Bacterial Biofilm Formation. In: Hosseini, M., Karapanagiotis, I. (eds) Materials with Extreme Wetting Properties. Springer, Cham. https://doi.org/10.1007/978-3-030-59565-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-59565-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59564-7
Online ISBN: 978-3-030-59565-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)