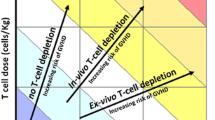
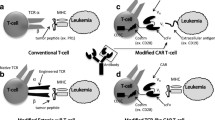
Abstract
Cellular therapy is an integral part of cancer immunotherapy, which is now considered as the fourth pillar of cancer treatment after surgery, chemotherapy, and radiotherapy. Allogeneic hematopoietic cell transplantation (HCT) is considered the most classic example of cellular immunotherapy. Post-transplant interplay between host and donor immune reactive cells plays a major role in graft-versus-leukemia (GvL) effect and graft-versus-host disease (GvHD). Immune reconstitution of the T-cell repertoire has major implications for relapsed disease, GvHD, and post-transplant viral infections. This chapter will focus on the exciting development in cellular therapy in the context of HCT and cell therapy including discussion of the diverse repertoire of T-cell products, specifically donor lymphocyte infusion (DLI), regulatory T cells, cytotoxic T lymphocytes for viral infections, and T-cell receptor gene-modified T cells. In addition, the underlying pathophysiology and mechanism of action of cell-mediated effects with a focus on evidence from clinical studies elaborating the indications, efficacy ,and safety of these T-cell-mediated therapies will be discussed, along with a brief overview of future directions and clinical trials exploring the potential of novel cell-mediated therapies in post-transplant settings.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Peggs KS, Mackinnon S. Cellular therapy: donor lymphocyte infusion. Curr Opin Hematol. 2001;8(6):349–54.
Guillaume T, Porcheron S, Audat F, et al. Prophylactic, preemptive and curative use of donor lymphocyte infusion in patients undergoing allogeneic stem cell transplantation: guidelines of the SFGM-TC. Pathol Biol. 2014;62(4):193–6.
Castagna L, Sarina B, Bramanti S, et al. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfus Apher Sci. 2016;54(3):345–55.
Nikiforow S, Alyea EP. Maximizing GVL in allogeneic transplantation: role of donor lymphocyte infusions. Hematology Am Soc Hematol Educ Program. 2014;2014(1):570–5.
Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008;21(2):205–22.
Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11(4):473–87.
Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev. 2013;27(1):55–62.
El-Jurdi N, Reljic T, Kumar A, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy. 2013;5(5):457–66.
Orti G, Barba P, Fox L, et al. Donor lymphocyte infusions in AML and MDS: enhancing the graft-versus-leukemia effect. Exp Hematol. 2017;48:1–11.
Stamouli M, Gkirkas K, Tsirigotis P. Strategies for improving the efficacy of donor lymphocyte infusion following stem cell transplantation. Immunotherapy. 2016;8(1):57–68.
Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7.
Uhl FM, Zeiser R. Regulatory T cells: broadening applicability. Cell and gene therapy. Perales MA, Abutalib SA, Bollard C. Chapter 9, pages 159–180 Series: Advances and Controversies in Hematopoietic Cell Transplantation and Cell Therapy. Series Eds. Abutalib SA, Armitage JO. Springer Switzerland 2019.
Belizário JE, Brandão W, Rossato C, Peron JP. Thymic and postthymic regulation of naïve CD4(+) T-cell lineage fates in humans and mice models. Mediat Inflamm. 2016;2016:9523628.
Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3.
Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13.
Pandiyan P, Zheng L, Ishihara S, et al. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–62.
Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58.
Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9.
Chen w JW, Hardegen N, et al. Conversion of peripheral CD4+CD25−naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86.
Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–56.
Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65.
Zeiser R, Nguyen VH, Hou JZ, et al. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood. 2007;109(5):2225–33.
Pierini A, Colonna L, Alvarez M, et al. Donor requirements for regulatory T cell suppression of murine graft-versus-host disease. J Immunol. 2015;195(1):347–55.
Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8.
Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–51.
Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8.
Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130–7.
Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379–86.
Zandvliet ML, van Liempt E, Jedema I, et al. Co-ordinated isolation of CD8(+) and CD4(+) T cells recognizing a broad repertoire of cytomegalovirus pp65 and IE1 epitopes for highly specific adoptive immunotherapy. Cytotherapy. 2010;12(7):933–44.
Withers B, Blyth E, Clancy LE, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017;1(24):2193–205.
Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134(1):64–76.
Neuenhahn M, Albrecht J, Odendahl M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after Allo-HSCT. Leukemia. 2017;31(10):2161–71.
Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–58.
Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–44.
Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375–7.
Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–406.
Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57.
Peggs KS, Verfuerth S, Pizzey A, et al. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis. 2009;49(12):1851–60.
Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(6):707–14.
Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–22.
Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–7.
Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–9.
Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–6.
Ma CK, Blyth E, Clancy L, et al. Addition of varicella zoster virus-specific T cells to cytomegalovirus, Epstein-Barr virus and adenovirus tri-specific T cells as adoptive immunotherapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Cytotherapy. 2015;17(10):1406–20s.
Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6(242):242ra83.
Hanley PJ, Melenhorst JJ, Nikiforow S, et al. CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo. Sci Transl Med. 2015;7(285):285ra63.
Hanley PJ. Build a bank: off-the-shelf virus-specific T cells. Biol Blood Marrow Transplant. 2018;24(12):e9–e10.
Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35(31):3547–57.
Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(9):1163–72.
O’Reilly RJ, Prockop S, Hasan AN, et al. Virus-specific T-cell banks for 'off the shelf' adoptive therapy of refractory infections. Bone Marrow Transplant. 2016;51(9):1163–72.
Withers B, Clancy L, Burgess J, et al. Establishment and operation of a third-party virus-specific T cell bank within an allogeneic stem cell transplant program. Biol Blood Marrow Transplant. 2018;24(12):2433–42. https://doi.org/10.1016/j.bbmt.2018.08.024.
Riaz N, Morris L, Havel JJ, et al. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28(8):411–9.
Morgan RA, Dudley JR, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9.
Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–21.
Morgan RA, Chinnasamy N, Abate-Daga A, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–51.
Van den Berg JH, Gomez-Eerland R, Van de Wiel B, et al. Case report of a fatal serious adverse event upon administration of T cells transduced with a MART-1-specific T-cell receptor. Mol Ther. 2015;23(9):1541–50.
Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46.
Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6.
van den Berg JH, Gomez-Eerland R, van de Wiel B, et al. Case report of a fatal serious adverse event upon administration of T cells transduced with a MART-1-specific T-cell receptor. Mol Ther. 2015;23(9):1541–50.
Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71.
Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014;5:235.
Further Readings
Donor Lymphocyte Infusion
Castagna L, Sarina B, Bramanti S, et al. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfus Apher Sci. 2016;54(3):345–55.
Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev. 2013;27(1):55–62.
Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109(11):4698–707.
De Vos J, Baudoux E, Bay JO, et al. Prophylactic, preemptive and curative use of donor lymphocyte infusion in patients undergoing allogeneic stem cell transplantation: guidelines of the SFGM-TC. Bull Cancer. 2018.10.002.
El-Jurdi N, Reljic T, Kumar A, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy. 2013;5(5):457–66.
Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008;21(2):205–22.
Goldsmith SR, Slade M, DiPersio JF, et al. Donor-lymphocyte infusion following haploidentical hematopoietic cell transplantation with peripheral blood stem cell grafts and PTCy. Bone Marrow Transplant. 2017;52(12):1623–8.
Miller JS, Warren EH, van den Brink MR, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biology underlying recurrence of malignant disease following allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant. 2010;16(5):565–86.
Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(3):314–8.
Regulatory T (Treg) Cells
Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7.
Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8.
Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft vs. host disease. Blood. 2016;128(1):130–7.
Mohty M, Gaugler B, Faucher C, et al. Recovery of lymphocyte and dendritic cell subsets following reduced intensity allogeneic bone marrow transplantation. Hematology. 2002;7(3):157–64.
Pandiyan P, Zheng L, Ishihara S, et al. CD4 + CD25 + Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–62.
Pierini A, Colonna L, Alvarez M, et al. Donor requirements for regulatory T cell suppression of murine graft-versus-host disease. J Immunol. 2015;195(1):347–55.
Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8.
Whangbo JS, Kim HT, Nikiforow S, et al. Functional analysis of clinical response to low-dose IL-2 in patients with refractory chronic graft-versus-host disease. Blood Adv. 2019;3(7):984–94.
Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21(3):579–96.
Cytotoxic T Lymphocytes for Viral Infections
Baugh KA, Tzannou I, Leen AM. Infusion of cytotoxic T lymphocytes for the treatment of viral infections in hematopoetic stem cell transplant patients. Curr Opin Infect Dis. 2018;31(4):292–300.
Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76.
Hanley PJ. Build a bank: off-the-shelf virus-specific T cells. Biol Blood Marrow Transplant. 2018;24(12)
Neuenhahn M, Albrecht J, Odendahl M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–71.
Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, epstein-barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35:3547–57.
Withers B, Blyth E, Clancy LE, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017;1:2193–205.
T-Cell Receptors-Gene-Modified T Cells for Cancer Treatment
Duong CP, Yong CS, Kershaw MH, et al. Cancer immunotherapy utilizing gene-modified T-cells: from the bench to the clinic. Mol Immunol. 2015;67(2 Pt A):46–57.
Gilham DE, Anderson J, Bridgeman JS, et al. Adoptive T-cell therapy for cancer in the United Kingdom: a review of activity for the British Society of Gen and Cell Therapy annual meeting 2015. Hum Gene Ther. 2015;26:276–85.
Rapoport AP, Yared JA. T-cell receptor (TCR)-gene modified T-cells for cancer – methods, data and challenges. Springer. Advances & controversies in hematopoietic cell transplants and cell therapy. Series editors: Syed A. Abutalib M.D. & James O. Armitage M.D. Volume 3. Cell and gene therapy. Volume editors – Miguel-Angel Perales M.D., Syed A. Abutalib MD & Catherine Bollard MD 10.1007/978-3-319-54368-0.
Thomas S, Stauss HJ, Morris EC. Molecular immunology lessons from therapeutic T-cell receptor gene transfer. Immunology. 2010;129(2):170–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hashmi, H., Majhail, N., Abutalib, S.A., Rapoport, A.P., Yared, J.A. (2021). T-Cell Therapeutics: Donor Lymphocyte Infusion, Cytotoxic T-Lymphocyte Infusion, and Other Non-CAR T-Cell Therapies. In: Maziarz, R.T., Slater, S.S. (eds) Blood and Marrow Transplant Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-53626-8_55
Download citation
DOI: https://doi.org/10.1007/978-3-030-53626-8_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53625-1
Online ISBN: 978-3-030-53626-8
eBook Packages: MedicineMedicine (R0)