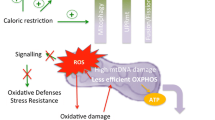
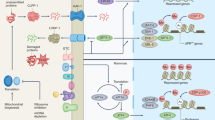
Abstract
The literature refers to the mitochondrion as the powerhouse of the cell, since this organelle is the primary source of energy production in the eukaryotic cells. However, mitochondria are not simple machines that produce energy. For instance, recent studies have proposed that mitochondria are a significant hub among several signaling pathways that regulate survival, immunity, and proteostasis that are also strongly associated with aging. Therefore, the study of mitochondrial function, which involves metabolism, distribution within the cell, and dynamics (fission, fusion, and mitophagy), has become relevant in the aging field. Moreover, the mitochondria are also the principal producers of reactive oxygen species, so mitochondrial dysfunction is an important hallmark of aging. In this chapter, we will analyze the role of mitochondrial dynamics and metabolism that, upon failure, lead to the organelle dysfunction, and their relationship with the aging process and age-related diseases. Finally, we will describe a few examples of treatments that have been used in order to target mitochondria and restore mitochondrial function.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ∆Ψm:
-
Mitochondrial membrane potential
- AD:
-
Alzheimer’s disease
- ATP:
-
Adenosine triphosphate
- BMI:
-
Body mass index
- DRP1:
-
Dynamin-related protein 1
- ETC:
-
Electron transport chain
- FA:
-
Fatty acids
- FAO:
-
Fatty acid oxidation
- Fis1:
-
Mitochondrial fission protein 1
- IMM:
-
Inner mitochondrial membrane
- MFF:
-
Mitochondrial fission factor
- MiD:
-
Mitochondrial division factors
- MPTP:
-
Mitochondrial permeability transition pore
- MRC:
-
Mitochondrial respiratory chain
- mtDNA:
-
Mitochondrial deoxyribonucleic acid
- mtUPR:
-
Mitochondrial unfolded protein response
- NRF2:
-
Nuclear respiratory factor 2
- OMM:
-
Outer mitochondrial membrane
- OxPhos:
-
Oxidative phosphorylation
- PARL:
-
Presenilin-associated rhomboid-like
- PD:
-
Parkinson’s disease
- PGC-1α :
-
Peroxisome proliferator-activated receptor γ
- Pol-ˠ:
-
Polymerase gamma
- PUFAs:
-
Polyunsaturated fatty acids
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- TCA:
-
Tricarboxylic acid cycle
- TFAM:
-
Mitochondrial transcription factor A
References
Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world – what do we know? Lancet. 2015;385:484–6.
Shadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Philos Trans R Soc Lond Ser B Biol Sci. 2011;366:2297–306.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Webb M, Sideris DP, Biddle M. Modulation of mitochondrial dysfunction for treatment of disease. Bioorg Med Chem Lett. 2019;29:1270–7.
Salatto CT, Miller RA, Cameron KO, et al. Selective activation of AMPK β 1-containing isoforms improves kidney function in a rat model of diabetic nephropathy. J Pharmacol Exp Ther. 2017;361:303–11.
Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865.
Sivapathasuntharam C, Sivaprasad S, Hogg C, Jeffery G. Improving mitochondrial function significantly reduces the rate of age related photoreceptor loss. Exp Eye Res. 2019;185:107691.
Payne, B. A., & Chinnery, P. F. Mitochondrial dysfunction in aging: much progress but many unresolved questions. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2015;1847(11):1347–53.
Sastre J, Pallardó FV, Plá R, Pellín A, Juan G, O’Connor JE, Estrela JM, Miquel J, Viña J. Aging of the liver: age-associated mitochondrial damage in intact hepatocytes. Hepatology. 1996;24:1199–205.
Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park J-Y, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci U S A. 1997;94:3064–9.
Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, Attardi G. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J. 2003;17:1706–8.
Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev. 1999;111:39–47.
Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–9.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Takamatsu C, Umeda S, Ohsato T, Ohno T, Abe Y, Fukuoh A, Shinagawa H, Hamasaki N, Kang D. Regulation of mitochondrial D-loops by transcription factor a and single-stranded DNA-binding protein. EMBO Rep. 2002;3:451–6.
Pinto M, Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med. 2015;85:250–8.
DeBalsi KL, Hoff KE, Copeland WC. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res Rev. 2017;33:89–104.
Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM 3rd, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–70.
Akhmedov AT, Marín-García J. Mitochondrial DNA maintenance: an appraisal. Mol Cell Biochem. 2015;409:283–305.
Harman D. Aging: a theory based on free radical and radiation chemistry. Berkeley: University of California Radiation Laboratory; 1955.
Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA–deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79:469–80.
Taylor RW, Barron MJ, Borthwick GM, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest. 2003;112:1351–60.
Booth LN, Brunet A. The aging epigenome. Mol Cell. 2016;62:728–44.
Pustylnikov S, Costabile F, Beghi S, Facciabene A. Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Transl Res. 2018;202:35–51.
Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–84.
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5.
Romanello V, Scalabrin M, Albiero M, Blaauw B, Scorrano L, Sandri M. Inhibition of the fission machinery mitigates OPA1 impairment in adult skeletal muscles. Cell. 2019;8:597.
Misgeld T, Schwarz TL. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron. 2017;96:651–66.
Kornfeld OS, Qvit N, Haileselassie B, Shamloo M, Bernardi P, Mochly-Rosen D. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Sci Rep. 2018;8:14034.
Malka F et al. Separate fusion of outer and inner mitochondrial membranes. PubMed – NCBI. https://www.ncbi.nlm.nih.gov/pubmed/16113651. Accessed 5 Jul 2019.
Frezza C, Cipolat S, Martins de Brito O, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–89.
Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–6.
Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77.
Wang Y, Qin Z-H. Coordination of autophagy with other cellular activities. Acta Pharmacol Sin. 2013;34:585–94.
Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501.
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407.
Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G. Autophagy in cardiovascular aging. Circ Res. 2018;123:803–24.
Linton PJ et al. This old heart: cardiac aging and autophagy. PubMed – NCBI. https://www.ncbi.nlm.nih.gov/pubmed/25543002. Accessed 5 Jul 2019.
**ao B, Sanders MJ, Carmena D, et al. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017.
Zhuang N, Li L, Chen S, Wang T. PINK1-dependent phosphorylation of PINK1 and Parkin is essential for mitochondrial quality control. Cell Death Dis. 2016;7:e2501.
Sekine S, Youle RJ. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 2018;16:2.
Narendra DP, ** SM, Tanaka A, Suen D-F, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298.
Zhou C, Huang Y, Shao Y, May J, Prou D, Perier C, Dauer W, Schon EA, Przedborski S. The kinase domain of mitochondrial PINK1 faces the cytoplasm. Proc Natl Acad Sci U S A. 2008;105:12022–7.
Vives-Bauza C, Zhou C, Huang Y, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–83.
Thai PN, Seidlmayer LK, Miller C, Ferrero M, Dorn GW II, Schaefer S, Bers DM, Dedkova EN. Mitochondrial quality control in aging and heart failure: influence of ketone bodies and Mitofusin-stabilizing peptides. Front Physiol. 2019;10:382.
Shiota, T., Traven, A., & Lithgow, T. Mitochondrial biogenesis: cell-cycle-dependent investment in making mitochondria. Current Biology. 2015;25(2):R78–R80.
Bouchez C, Devin A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cell. 2019. https://doi.org/10.3390/cells8040287.
Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24.
Pickrell, A. M., & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–73.
Wang H, Song P, Du L, et al. Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem. 2011;286:11649–58.
Exner N, Treske B, Paquet D, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by Parkin. J Neurosci. 2007;27:12413–8.
Schapira, A. H. Etiology of Parkinson’s disease. Neurology. 2006; 66(10 suppl 4), S10–S23.
Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis. 2006;9:119–26.
Reddy PH, Tripathi R, Troung Q, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–49.
Cho D-H, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–5.
Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–301.
Iqbal S, Ostojic O, Singh K, Joseph A-M, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013;48:963–70.
Tezze C, Romanello V, Desbats MA, et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 2017;25:1374–89.e6.
Favaro G, Romanello V, Varanita T, et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat Commun. 2019;10:2576.
Buso A, Comelli M, Picco R, et al. Mitochondrial adaptations in elderly and young men skeletal muscle following 2 weeks of bed rest and rehabilitation. Front Physiol. 2019;10:474.
Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–60.
Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–9.
Niemann B, Chen Y, Teschner M, Li L, Silber R-E, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol. 2011;57:577–85.
Niemann B, Pan R, Teschner M, Boening A, Silber R-E, Rohrbach S. Age and obesity-associated changes in the expression and activation of components of the AMPK signaling pathway in human right atrial tissue. Exp Gerontol. 2013;48:55–63.
Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;8:349–69.
Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proceedings of the National Academy of Sciences. 2011;108(23):9572–77.
Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–509.
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 2017;40:151–66.
Srivastava, S. The mitochondrial basis of aging and age-related disorders. Genes. 2017;8(12):398.
Kitada M, Ogura Y, Monno I, Koya D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine. 2019;43:632–40.
Herst PM, Rowe MR, Carson GM, Berridge MV. Functional mitochondria in health and disease. Front Endocrinol. 2017;8:296.
López-Lluch G, Hernández-Camacho JD, Fernández-Ayala DJM, Navas P. Mitochondrial dysfunction in metabolism and ageing: shared mechanisms and outcomes? Biogerontology. 2018;19:461–80.
Long YC, Tan TMC, Takao I, Tang BL. The biochemistry and cell biology of aging: metabolic regulation through mitochondrial signaling. Am J Physiol Endocrinol Metab. 2014;306:E581–91.
Zhang R, Wang Y, Ye K, Picard M, Gu Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics. 2017;18:890.
Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech Ageing Dev. 2010;131:451–62.
Liu D, Li H, Lu J, Bai Y. Tissue-specific implications of mitochondrial alterations in aging. Front Biosci. 2013;5:734–47.
Nissanka N, Moraes CT. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592:728–42.
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–17.
Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21.
Carter HN, Chen CCW, Hood DA. Mitochondria, muscle health, and exercise with advancing age. Physiology. 2015;30:208–23.
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci. 2005;102:5618–23.
Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, Studenski SA, Spencer RG, Ferrucci L. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16:461–8.
Gueugneau M, Coudy-Gandilhon C, Gourbeyre O, et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genomics. 2014;15:1165.
Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:1–20.
Distefano G, Goodpaster BH. Effects of exercise and aging on skeletal muscle. Cold Spring Harb Perspect Med. 2018. https://doi.org/10.1101/cshperspect.a029785.
Lalia AZ, Dasari S, Robinson MM, Abid H, Morse DM, Klaus KA, Lanza IR. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging. 2017;9:1096–129.
Kent JA, Fitzgerald LF. In vivo mitochondrial function in aging skeletal muscle: capacity, flux, and patterns of use. J Appl Physiol. 2016;121:996–1003.
Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63.
Mercken EM, Crosby SD, Lamming DW, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–51.
Potes Y, Pérez-Martinez Z, Bermejo-Millo JC, et al. Overweight in the elderly induces a switch in energy metabolism that undermines muscle integrity. Aging Dis. 2019;10:217–30.
Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Molina AJA. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016;10:65–77.
Chaudhary KR, El-Sikhry H, Seubert JM. Mitochondria and the aging heart. J Geriatr Cardiol. 2011;8:159–67.
Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res. 2016;118:1593–611.
Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–6.
Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–9.
Wei Y-H, Wu S-B, Ma Y-S, Lee H-C. Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging. Chang Gung Med J. 2009;32:113–32.
Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–9.
Gómez LA, Monette JS, Chavez JD, Maier CS, Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys. 2009;490:30–5.
Emelyanova L, Preston C, Gupta A, et al. Effect of aging on mitochondrial energetics in the human atria. J Gerontol A Biol Sci Med Sci. 2018;73:608–16.
Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Changes in the mitochondrial permeability transition pore in aging and age-associated diseases. Mech Ageing Dev. 2013;134:1–9.
Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res Int. 2014;2014:238463.
Müller-Höcker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart – an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–73.
Wegrzyn J, Potla R, Chwae J-Y, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7.
Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108–22.
Selvaraji S, Poh L, Natarajan V, Mallilankaraman K, Arumugam TV. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond Med. 2019;2:30–9.
Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–21.
Zhu X-H, Lu M, Lee B-Y, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112:2876–81.
Blass JP, Sheu RK, Gibson GE. Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise. Ann N Y Acad Sci. 2000;903:204–21.
Liang WS, Reiman EM, Valla J, et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105:4441–6.
Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM. Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol. 2016;79:366–78.
Reeve A, Meagher M, Lax N, Simcox E, Hepplewhite P, Jaros E, Turnbull D. The impact of pathogenic mitochondrial DNA mutations on substantia nigra neurons. J Neurosci. 2013;33:10790–801.
Lowell BB. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7.
Acknowledgments
This work was supported by CONACYT grant FOSSIS-272256, as well as the “Red Temática de Investigación en Salud y Desarrollo Social” from CONACYT. Morales-Rosales is a CONACYT scholarship holder (CVU 718720 and scholarship number 702613). Authors thank Ariadna Guerrero-Cruz for the artwork.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morales-Rosales, S.L., Rivero-Segura, N.A., Königsberg, M. (2020). Mitochondrial Function in Aging. In: Gomez-Verjan, J., Rivero-Segura, N. (eds) Clinical Genetics and Genomics of Aging. Springer, Cham. https://doi.org/10.1007/978-3-030-40955-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-40955-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40954-8
Online ISBN: 978-3-030-40955-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)