Abstract
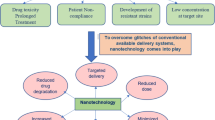
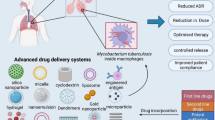
Nanoparticle-based delivery systems represent a promising nano medications to deliver a therapeutic agent, selectively and effectively, to a specific tissue or organ in the body; thus treating chronic diseases such as tuberculosis. The delivery of first-line and second-line antituberculosis drugs, using synthetic or natural polymeric carriers, has been extensively reported as a potential intermittent chemotherapy. In addition to the prolonged drug release, this delivery system can enhance the therapeutic efficacy, reduce dosing frequency and side effects, and increase the possibility of selecting different routes of chemotherapy and targeting the site of infection. The choice of carrier, system stability, toxicity and production capacity are the main considerations during the development of such system. Regardless of the obstacles, the nano drug delivery have systems shown a promising effectiveness in treating TB.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdulla, J. M., Tan, Y. T., & Darwis, Y. (2010). Rehydrated lyophilized rifampicin-loaded mPEGDSPE formulations for nebulization. AAPS PharmSciTech, 11, 663–671.
Agarwal, A., Kandpal, H., Gupta, H. P., Singh, N. B., & Gupta, C. M. (1994). Tuftsin-bearing liposomes as rifampin vehicles in treatment of tuberculosis in mice. Antimicrobial Agents and Chemotherapy, 38, 588–593.
Ahmad, S., & Mokaddas, E. (2014). Current status and future trends in the diagnosis and treatment of drug-susceptible and multidrug-resistant tuberculosis. Journal of Infection and Public Health, 7, 75–91.
Ahmad, Z., Sharma, S., & Khuller, G. K. (2005). Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. International Journal of Antimicrobial Agents, 26, 298–303.
Ahmed, E. M. (2015). Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research, 6, 105–121.
Al-Hallak, M. H. D. K., Sarfraz, M. K., Azarmi, S., Roa, W. H., Finlay, W. H., & Rouleau, C. (2012). Distribution of effervescent inhalable nanoparticles after pulmonary delivery: An in vivo study. Therapeutic Delivery, 3, 725–773.
Amani, A., Amini, M. A., Ali, H. S., & York, P. (2011). Alternatives to conventional suspensions for pulmonary drug delivery by nebulisers: A review. Journal of Pharmaceutical Sciences, 100, 4563–4570.
Anabousi, S., Kleemann, E., Bakowsky, U., Kissel, T., Schmehl, T., Gessler, T., et al. (2006). Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. Journal of Nanoscience and Nanotechnology, 6, 3010–3016.
Andersen, P., Munk, M. E., Pollock, J. M., & Doherty, T. M. (2000). Specific immune-based diagnosis of tuberculosis. Lancet, 356, 1099–1104.
Andrade, F., Rafael, D., Videira, M., Ferreira, D., Sosnik, A., & Sarmento, B. (2013). Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Advanced Drug Delivery Reviews, 65, 1816–1827.
Asadi Gharabaghi, M. (2012). Cutaneous tuberculosis caused by isoniazid-resistant Mycobacterium tuberculosis. BMJ Case Reports. (2012).
Azarmi, S., Lobenberg, R., Roa, W. H., Tai, S., & Finlay, W. H. (2008). Formulation and in vivo evaluation of effervescent inhalable carrier particles for pulmonary delivery of nanoparticles. Drug Development and Industrial Pharmacy, 34, 943–947.
Bajpai, A. K., & Gupta, R. (2011). Magnetically mediated release of ciprofloxacin from polyvinyl alcohol based superparamagnetic nanocomposites. Journal of Materials Science. Materials in Medicine, 22, 357–369.
Bangham, A. D. (1993). Liposomes: The Babraham connection. Chemistry and Physics of Lipids, 64, 275–285.
Barry, C. E., III, Boshoff, H. I., Dartois, V., Dick, T., Ehrt, S., Flynn, J., et al. (2009). The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nature Reviews Microbiology, 7, 845–855.
Beck-Broichsitter, M., Merkel, O. M., & Kissel, T. (2012). Controlled pulmonary drug and gene delivery using polymeric nano-carriers. Journal of Controlled Release, 161, 214–224.
Behr, M. A., Warren, S. A., Salamon, H., Hopewell, P. C., Ponce de Leon, A., Daley, C. L., et al. (1999). Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet, 353, 444–449.
Bellini, R. G., Guimarães, A. P., Pacheco, M. A. C., Dias, D. M., Furtado, V. R., de Alencastro, R. B., et al. (2015). Association of the anti-tuberculosis drug rifampicin with a PAMAM dendrimer. Journal of Molecular Graphics and Modelling, 60, 34–42.
Booysen, L. L., Kalombo, L., Brooks, E., Hansen, R., Gilliland, J., Gruppo, V., et al. (2013). In vivo/in vitro pharmacokinetic and pharmacodynamic study of spray-dried poly-(dl-lactic-co-glycolic) acid nanoparticles encapsulating rifampicin and isoniazid. International Journal of Pharmaceutics, 444, 10–17.
Bosquillon, C., Lombry, C., Preat, V., & Vanbever, R. (2001). Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. Journal of Controlled Release, 70, 329–339.
Breslauer, D. N., Maamari, R. N., Switz, N. A., Lam, W. A., & Fletcher, D. A. (2009). Mobile phone based clinical microscopy for global health applications. PLoS One 2009; 4.
Buijtels, P. C., Willemse-Erix, H. F., Petit, P. L., Endtz, H. P., Puppels, G. J., Verbrugh, H. A., et al. (2008). Rapid identification of mycobacteria by Raman spectroscopy. Journal of Clinical Microbiology, 46, 961–965.
Caon, T., Campos, C. E., Simoes, C. M., & Silva, M. A. (2015). Novel perspectives in the tuberculosis treatment: Administration of isoniazid through the skin. International Journal of Pharmaceutics, 494, 463–470.
Cattamanchi, A., Smith, R., Steingart, K. R., Metcalfe, J. Z., Date, A., Coleman, C., et al. (2011). Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: A systematic review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes, 56, 230–238.
Chan, J. G., Chan, H. K., Prestidge, C. A., Denman, J. A., Young, P. M., & Traini, D. (2013). A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. European Journal of Pharmaceutics and Biopharmaceutics, 83, 285–292.
Chen, T., Li, Q., Guo, L., Yu, L., Li, Z., Guo, H., et al. (2016). Lower cytotoxicity, high stability, and long-term antibacterial activity of a poly(methacrylic acid)/isoniazid/rifampin nanogel against multidrug-resistant intestinal Mycobacterium tuberculosis. Materials Science and Engineering C: Materials for Biological Applications, 58, 659–665.
Chen, J., Zhang, R., Wang, J., Liu, L., Zheng, Y., Shen, Y., et al. (2011). Interferon-gamma release assays for the diagnosis of active tuberculosis in HIV-infected patients: A systematic review and meta-analysis. PLoS ONE, 6, e26827.
Cheow, W. S., & Hadinoto, K. (2010). Enhancing encapsulation efficiency of highly water-soluble antibiotic in poly(lactic-co-glycolic acid) nanoparticles: Modifications of standard nanoparticle preparation methods. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 370, 79–86.
Cheow, W. S., & Hadinoto, K. (2011). Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids and Surfaces B: Biointerfaces, 85, 214–220.
Chimote, G., & Banerjee, R. (2005). Effect of antitubercular drugs on dipalmitoylphosphatidylcholine monolayers: Implications for drug loaded surfactants. Respiratory Physiology & Neurobiology, 145, 65–77.
Chimote, G., & Banerjee, R. (2009). Evaluation of antitubercular drug-loaded surfactants as inhalable drug-delivery systems for pulmonary tuberculosis. Journal of Biomedical Materials Research, 89, 281–292.
Chono, S., Kaneko, K., Yamamoto, E., Togami, K., & Morimoto, K. (2010). Effect of surface mannose modification on aerosolized liposomal delivery to alveolar macrophages. Drug Development and Industrial Pharmacy, 36, 102–107.
Choonara, Y. E., Pillay, V., Ndesendo, V. M. K., du Toit, L. C., Kumar, P., Khan, R. A., et al. (2011). Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids and Surfaces B: Biointerfaces, 87, 243–254.
Chow, A. H., Tong, H. H., Chattopadhyay, P., & Shekunov, B. Y. (2007). Particle engineering for pulmonary drug delivery. Pharmaceutical Research, 24, 411–437.
Chuan, J., Li, Y., Yang, L., Sun, X., Zhang, Q., Gong, T., et al. (2013). Enhanced rifampicin delivery to alveolar macrophages by solid lipid nanoparticles. Journal of Nanoparticle Research, 15, 1–9.
Chun, A. L. (2009). Nanoparticles offer hope for TB detection. Nature Nanotechnology, 4, 698–699.
Clemens, D. L., Lee, B. Y., Xue, M., Thomas, C. R., Meng, H., Ferris, D., et al. (2012). Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrobial Agents and Chemotherapy, 56, 2535–2545.
Cobelens, F. G., Egwaga, S. M., van Ginkel, T., Muwinge, H., Matee, M. I., & Borgdorff, M. W. (2006). Tuberculin skin testing in patients with HIV infection: Limited benefit of reduced cutoff values. Clinical Infectious Diseases, 43, 634–639.
Costa, P., Amaro, A., Botelho, A., Inacio, J., & Baptista, P. V. (2010). Gold nanoprobe assay for the identification of mycobacteria of the Mycobacterium tuberculosis complex. Clinical Microbiology & Infection, 16, 1464–1469.
Costa, A., Sarmento, B., & Seabra, V. (2015). Targeted drug delivery systems for lung macrophages. Current Drug Targets, 16, 1565–1581.
Dames, P., Gleich, B., Flemmer, A., Hajek, K., Seidl, N., Wiekhorst, F., et al. (2007). Targeted delivery of magnetic aerosol droplets to the lung. Nature Nanotechnology, 2, 495–499.
Dartois, V. (2014). The path of anti-tuberculosis drugs: From blood to lesions to mycobacterial cells. Nature Reviews Microbiology, 12, 159–167.
Das, S., Tucker, I., & Stewart, P. (2015). Inhaled dry powder formulations for treating tuberculosis. Current Drug Delivery, 12, 26–39.
de Faria, T. J., Roman, M., de Souza, N. M., De Vecchi, R., de Assis, J. V., dos Santos, A. L., et al. (2012). An isoniazid analogue promotes Mycobacterium tuberculosis-nanoparticle interactions and enhances bacterial killing by macrophages. Antimicrobial Agents and Chemotherapy, 56, 2259–2267.
Deol, P., Khuller, G. K., & Joshi, K. (1997). Therapeutic efficacies of isoniazid and rifampin encapsulated in lung-specific stealth liposomes against Mycobacterium tuberculosis infection induced in mice. Antimicrobial Agents and Chemotherapy, 41, 1211–1214.
Desai, T. R., Hancock, R. E. W., & Finlay, W. H. (2002a). A facile method of delivery of liposomes by nebulization. Journal of Controlled Release, 84, 69–78.
Desai, T. R., Wong, J. P., Hancock, R. E. W., & Finlay, W. H. (2002b). A novel approach to the pulmonary delivery of liposomes in dry powder form to eliminate the deleterious effects of milling. Journal of Pharmaceutical Sciences, 91, 482–491.
Dheda, K., van Zyl Smit, R., Badri, M., & Pai, M. (2009). T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: Clinical utility in high-burden vs. low-burden settings. Current Opinion in Pulmonary Medicine, 15, 188–200.
Diaz-Gonzalez, M., Gonzalez-Garcia, M. B., & Costa-Garcia, A. (2005). Immunosensor for Mycobacterium tuberculosis on screen-printed carbon electrodes. Biosensors & Bioelectronics, 20, 2035–2043.
Douglas, J. G., & McLeod, M. J. (1999). Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clinical Pharmacokinetics, 37, 127–146.
du Toit, L. C., Pillay, V., & Danckwerts, M. P. (2006). Tuberculosis chemotherapy: Current drug delivery approaches. Respiratory Research, 7, 118.
Dunlap, N. E., Bass, J., Fujiwara, P., Hopewell, P., Horsburgh, C. R., & Salfinger, H. M. (2000). Diagnostic standards and classification of tuberculosis in adults and children. American Journal of Respiratory and Critical Care Medicine, 161, 1376–1395.
El-Gendy, N., Desai, V., & Berkland, C. (2010). Agglomerates of ciprofloxacin nanoparticles yield fine dry powder aerosols. Journal of Pharmaceutical Innovation, 5, 79–87.
Ely, L., Roa, W., Finlay, W. H., & Lobenberg, R. (2007). Effervescent dry powder for respiratory drug delivery. European Journal of Pharmaceutics and Biopharmaceutics, 65, 346–353.
Esmaeili, F., Hosseini-Nasr, M., Rad-Malekshahi, M., Samadi, N., Atyabi, F., & Dinarvand, R. (2007). Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomedicine, 3, 161–167.
Farhat, M., Greenaway, C., Pai, M., & Menzies, D. (2006). False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? International Journal of Tuberculosis and Lung Disease, 10, 1192–1204.
Feng, H., Zhang, L., & Zhu, C. (2013). Genipin crosslinked ethyl cellulose–chitosan complex microspheres for anti-tuberculosis delivery. Colloids and Surfaces B: Biointerfaces, 103, 530–537.
Ferron, G. A. (1994). Aerosol properties and lung deposition. European Respiratory Journal, 7, 1392–1394.
Ferron, G. A., Upadhyay, S., Zimmermann, R., & Karg, E. (2013). Model of the deposition of aerosol particles in the respiratory tract of the rat. II. Hygroscopic particle deposition. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 26, 101–119.
Finlay, W. H., & Wong, J. P. (1998). Regional lung deposition of nebulized liposome encapsulated ciprofloxacin. International Journal of Pharmaceutics, 167, 121–127.
Gao, L., Liu, G., Ma, J., Wang, X., Zhou, L., & Li, X. (2012). Drug nanocrystals: In vivo performances. Journal of Controlled Release, 160, 418–430.
Garg, T., Rath, G., & Goyal, A. K. (2015). Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artificial Cells, Nanomedicine, and Biotechnology, 44, 997–1001.
Gaur, P. K., Mishra, S., Gupta, V. B., Rathod, M. S., Purohit, S., & Savla, B. A. (2010). Targeted drug delivery of rifampicin to the lungs: Formulation, characterization, and stability studies of preformed aerosolized liposome and in situ formed aerosolized liposome. Drug Development and Industrial Pharmacy, 36, 638–646.
Gill, S., Löbenberg, R., Ku, T., Azarmi, S., Roa, W., & Prenner, E. J. (2007). Nanoparticles: Characteristics, mechanisms of action, and toxicity in pulmonary drug delivery—A review. Journal of Biomedical Nanotechnology, 3, 107–119.
Ginsberg, A. M. (2010). Tuberculosis drug development: Progress, challenges, and the road ahead. Tuberculosis, 90, 162–167.
Grenha, A., Seijo, B., & Remunan-Lopez, C. (2005). Microencapsulated chitosan nanoparticles for lung protein delivery. European Journal of Pharmaceutical Sciences, 25, 427–437.
Grosset, J. H., Singer, T. G., & Bishai, W. R. (2012). New drugs for the treatment of tuberculosis: Hope and reality. International Journal of Tuberculosis and Lung Disease, 16, 1005–1014.
Hanif, S. N., & Garcia-Contreras, L. (2012). Pharmaceutical aerosols for the treatment and prevention of tuberculosis. Frontiers in Cellular and Infection Microbiology, 2, 118.
He, F., Zhao, J., Zhang, L., & Su, X. (2003). A rapid method for determining Mycobacterium tuberculosis based on a bulk acoustic wave impedance biosensor. Talanta, 59, 935–941.
Hearn, M. J., & Cynamon, M. H. (2003). In vitro and in vivo activities of acylated derivatives of isoniazid against Mycobacterium tuberculosis. Drug Design and Discovery, 18, 103–108.
Hearn, M. J., Cynamon, M. H., Chen, M. F., Coppins, R., Davis, J., & Joo-On Kang, H. (2009). Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. European Journal of Medicinal Chemistry, 44, 4169–4178.
Hokey, D. A., & Misra, A. (2011). Aerosol vaccines for tuberculosis: A fine line between protection and pathology. Tuberculosis, 91, 82–85.
Homola, J. (2008). Surface plasmon resonance sensors for detection of chemical and biological species. Chemical Reviews, 108, 462–493.
Hong, S. C., Chen, H. X., Lee, J., Park, H. K., Kim, Y. S., Shin, H. C., et al. (2011). Ultrasensitive immunosensing of tuberculosis CFP-10 based on SPR spectroscopy. Sensors and Actuators B: Chemical, 156, 271–275.
Höök, F., Kasemo, B., Nylander, T., Fant, C., Sott, K., & Elwing, H. (2001). Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Analytical Chemistry, 73, 5796–5804.
Horváti, K., Bacsa, B., Kiss, É., Gyulai, G., Fodor, K., Balka, G., et al. (2014). Nanoparticle encapsulated lipopeptide conjugate of antitubercular drug isoniazid: In vitro intracellular activity and in vivo efficacy in a guinea pig model of tuberculosis. Bioconjugate Chemistry, 25, 2260–2268.
Horváti, K., Bacsa, B., Szabo, N., Fodor, K., Balka, G., Rusvai, M., et al. (2015). Antimycobacterial activity of peptide conjugate of pyridopyrimidine derivative against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Tuberculosis, 95, S207–S211.
Jain, D., & Banerjee, R. (2008). Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 86, 105–112.
Jain, S. K., Gupta, Y., Ramalingam, L., Jain, A., Jain, A., Khare, P., et al. (2010). Lactoseconjugated PLGA nanoparticles for enhanced delivery of rifampicin to the lung for effective treatment of pulmonary tuberculosis. Journal of Pharmaceutical Science and Technology, 64, 278–287.
Johnson, C. M., Pandey, R., Sharma, S., Khuller, G. K., Basaraba, R. J., Orme, I. M., et al. (2005). Oral therapy using nanoparticle-encapsulated antituberculosis drugs in guinea pigs infected with Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy, 49, 4335–4338.
Justo, O. R., & Moraes, A. M. (2003). Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Delivery, 10, 201–207.
Kabanov, A. V., & Vinogradov, S. V. (2009). Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angewandte Chemie International Edition, 48, 5418–5429.
Kajjari, P. B., Manjeshwar, L. S., & Aminabhavi, T. M. (2012). Novel pH- and temperature responsive blend hydrogel microspheres of sodium alginate and PNIPAAm-g-GG for controlled release of isoniazid. AAPS PharmSciTech, 13, 1147–1157.
Keijsers, R. R., Bovenschen, H. J., & Seyger, M. M. (2011). Cutaneous complication after BCG vaccination: Case report and review of the literature. Journal of Dermatological Treatment, 22, 315–318.
Kennedy, E. J. (2013). Biological drug products: Development and strategies. Hoboken: Wiley.
Kong, F., Zhou, F., Ge, L., Liu, X., & Wang, Y. (2012). Mannosylated liposomes for targeted gene delivery. International Journal of Nanomedicine, 7, 1079–1089.
Lee, J. Y. (2015). Diagnosis and treatment of extrapulmonary tuberculosis. Tuberculosis and Respiratory Diseases, 78, 47–55.
Lee, W., Loo, C., Traini, D., & Young, P. M. (2015). Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opinion on Drug Delivery, 12, 1009–1026.
Lee, H., Sun, E., Ham, D., & Weissleder, R. (2008). Chip-NMR biosensor for detection and molecular analysis of cells. Nature Medicine, 14, 869–874.
Li, X., Xue, M., Raabe, O. G., Aaron, H. L., Eisen, E. A., Evans, J. E., et al. (2015). Aerosol droplet delivery of mesoporous silica nanoparticles: A strategy for respiratory-based therapeutics. Nanomedicine, 11, 1377–1385.
Ling, D. I., Pai, M., Davids, V., Brunet, L., Lenders, L., Meldau, R., et al. (2011). Are interferon-gamma release assays useful for diagnosing active tuberculosis in a high-burden setting? European Respiratory Journal, 38, 649–656.
Malathi, S., & Balasubramanian, S. (2011). Synthesis of biodegradable polymeric nanoparticles and their controlled drug delivery for tuberculosis. Journal of Biomedical Nanotechnology, 7, 150–151.
Mamaeva, V., Sahlgren, C., & Lindén, M. (2013). Mesoporous silica nanoparticles in medicine—Recent advances. Advanced Drug Delivery Reviews, 65, 689–702.
Manion, J. A. R., Cape, S. P., McAdams, D. H., Rebits, L. G., Evans, S., & Sievers, R. E. (2012). Inhalable antibiotics manufactured through use of near-critical or supercritical fluids. Aerosol Science and Technology, 46, 403–410.
Martin, C. (2005). The dream of a vaccine against tuberculosis: New vaccines improving or replacing BCG? European Respiratory Journal, 26, 162–167.
Mazurek, G. H., Jereb, J., Lobue, P., Iademarco, M. F., Metchock, B., & Vernon, A. (2005). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recommendations and Reports, 54, 49–55.
Mehanna, M. M., Mohyeldin, S. M., & Elgindy, N. A. (2014). Respirable nanocarriers as a promising strategy for antitubercular drug delivery. Journal of Controlled Release, 187, 183–197.
Metcalfe, J. Z., Everett, C. K., Steingart, K. R., Cattamanchi, A., Huang, L., Hopewell, P. C., et al. (2011). Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: Systematic review and meta-analysis. Journal of Infectious Diseases, 204(Suppl. 4), S1120–S1129.
Misra, A., Hickey, A. J., Rossi, C., Borchard, G., Terada, H., Makino, K., et al. (2011). Inhaled drug therapy for treatment of tuberculosis. Tuberculosis, 91, 71–81.
Mitchison, D. A., & Fourie, P. B. (2010). The near future: Improving the activity of rifamycins and pyrazinamide. Tuberculosis, 90, 177–181.
Moghimi, S. M., Hunter, A. C., & Murray, J. C. (2005). Nanomedicine: Current status and future prospects. FASEB Journal, 19, 311–330.
Moretton, M. A., Hocht, C., Taira, C., & Sosnik, A. (2014). Rifampicin-loaded ‘flower-like’ polymeric micelles for enhanced oral bioavailability in an extemporaneous liquid fixed-dose combination with isoniazid. Nanomedicine (London), 9, 1635–1650.
Mouritsen, O. G. (2011). Model answers to lipid membrane questions. Cold Spring Harbor Perspectives in Biology, 3, a004622.
Muttil, P., Wang, C., & Hickey, A. J. (2009). Inhaled drug delivery for tuberculosis therapy. Pharmaceutical Research, 26, 2401–2416.
Nagel, T., Ehrentreich-Forster, E., Singh, M., Schmitt, K., Brandenburg, A., Berka, A., et al. (2008). Direct detection of tuberculosis infection in blood serum using three optical label-free approaches. Sensors and Actuators B: Chemical, 129, 934–940.
Nimje, N., Agarwal, A., Saraogi, G. K., Lariya, N., Rai, G., Agrawal, H., et al. (2009). Mannosylated nanoparticulate carriers of rifabutin for alveolar targeting. Journal of Drug Targeting, 17, 777–787.
Onoshita, T., Shimizu, Y., Yamaya, N., Miyazaki, M., Yokoyama, M., Fujiwara, N., et al. (2010). The behavior of PLGA microspheres containing rifampicin in alveolar macrophages. Colloids and Surfaces B: Biointerfaces, 76, 151–157.
Onozaki, I., & Raviglione, M. (2010). Stop** tuberculosis in the 21st century: Goals and strategies. Respirology, 15, 32–43.
Pai, N. P., & Pai, M. (2012). Point-of-care diagnostics for HIV and tuberculosis: Landscape, pipeline, and unmet needs. Discovery Medicine, 13, 35–45.
Pandey, R., & Ahmad, Z. (2011). Nanomedicine and experimental tuberculosis: Facts, flaws, and future. Nanomedicine, 7, 259–272.
Pandey, R., & Khuller, G. K. (2004). Chemotherapeutic potential of alginate–chitosan microspheres as anti-tubercular drug carriers. Journal of Antimicrobial Chemotherapy, 53, 635–640.
Pandey, R., & Khuller, G. K. (2005a). Antitubercular inhaled therapy: Opportunities, progress and challenges. Journal of Antimicrobial Chemotherapy, 55, 430–435.
Pandey, R., & Khuller, G. K. (2005b). Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis, 85, 227–234.
Pandey, R., Sharma, S., & Khuller, G. K. (2005). Oral solid lipid nanoparticle-based antitubercular chemotherapy. Tuberculosis, 85, 415–420.
Pandey, R., Sharma, A., Zahoor, A., Sharma, S., Khuller, G. K., & Prasad, B. (2003). Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. Journal of Antimicrobial Chemotherapy, 52, 981–986.
Patil, J. S., Devi, V. K., Devi, K., & Sarasija, S. (2015). A novel approach for lung delivery of rifampicin-loaded liposomes in dry powder form for the treatment of tuberculosis. Lung India, 32, 331–338.
Patil-Gadhe, A., & Pokharkar, V. (2014). Single step spray drying method to develop proliposomes for inhalation: A systematic study based on quality by design approach. Pulmonary Pharmacology & Therapeutics, 27, 197–207.
Peh, W. Y. X., Reimhult, E., Teh, H. F., Thomsen, J. S., & Su, X. (2007). Understanding ligand binding effects on the conformation of estrogen receptor α-DNA complexes: A combinational quartz crystal microbalance with dissipation and surface plasmon resonance study. Biophysical Journal, 92, 4415–4423.
Pham, D. D., Fattal, E., & Tsapis, N. (2015). Pulmonary drug delivery systems for tuberculosis treatment. International Journal of Pharmaceutics, 478, 517–529.
Pinheiro, M., Lima, J., & Reis, S. (2011). Liposomes as drug delivery systems for the treatment of TB. Nanomedicine, 6, 1413–1428.
Pitt, J. M., Blankley, S., McShane, H., & O’Garra, A. (2013). Vaccination against tuberculosis: How can we better BCG? Microbial Pathogenesis, 58, 2–16.
Pourshahab, P. S., Gilani, K., Moazeni, E., Eslahi, H., Fazeli, M. R., & Jamalifar, H. (2011). Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. Journal of Microencapsulation, 28, 605–613.
Prabakaran, D., Singh, P., Jaganathan, K. S., & Vyas, S. P. (2004). Osmotically regulated asymmetric capsular systems for simultaneous sustained delivery of anti-tubercular drugs. Journal of Controlled Release, 95, 239–248.
Prabhakar, N., Arora, K., Arya, S. K., Solanki, P. R., Iwamoto, M., Singh, H., et al. (2008). Nucleic acid sensor for M. tuberculosis detection based on surface plasmon resonance. Analyst, 133, 1587–1592.
Qurrat-ul-Ain, S., Sharma, G. K., & Khuller, S. K. (2003). Garg, alginate-based oral drug delivery system for tuberculosis: Pharmacokinetics and therapeutic effects. Journal of Antimicrobial Chemotherapy, 51, 931–938.
Radtke, M., Souto, E. B., & Muller, R. H. (2005). Nanostructured lipid carriers—A novel generation of solid lipid drug carriers. Pharmaceutical Technology Europe, 17, 45–50.
Ranjita, S., Loaye, A. S., & Khalil, M. (2011). Present status of nanoparticle research for treatment of tuberculosis. Journal of Pharmacy & Pharmaceutical Sciences, 14, 100–116.
Ren, J., He, F., Yi, S., & Cui, X. (2008). A new MSPQC for rapid growth and detection of Mycobacterium tuberculosis. Biosensors & Bioelectronics, 24, 403–409.
Roa, W. H., Azarmi, S., Al-Hallak, M. H., Finlay, W. H., Magliocco, A. M., & Lobenberg, R. (2011). Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. Journal of Controlled Release, 150, 49–55.
Rytting, E., Nguyen, J., Wang, X., & Kissel, T. (2008). Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opinion on Drug Delivery, 5, 629–639.
Santos, J. B., Figueiredo, A. R., Ferraz, C. E., Oliveira, M. H., Silva, P. G., & Medeiros, V. L. (2014). Cutaneous tuberculosis: Epidemiologic, etiopathogenic and clinical aspects—Part I. Anais Brasileiros de Dermatologia, 89, 219–228.
Sarkar, S., & Suresh, M. R. (2011). An overview of tuberculosis chemotherapy—A literature review. Journal of Pharmacy & Pharmaceutical Sciences, 14, 148–161.
Schütz, C. A., Juillerat-Jeanneret, L., Käuper, P., & Wandrey, C. (2011). Cell response to the exposure to chitosan–TPP//alginate nanogels. Biomacromolecules, 12, 4153–4161.
Semaan, R., Traboulsi, R., & Kanj, S. (2008). Primary Mycobacterium tuberculosis complex cutaneous infection: Report of two cases and literature review. International Journal of Infectious Diseases, 12, 472–477.
Sethi, T., & Agrawal, A. (2011). Structure and function of the tuberculous lung: Considerations for inhaled therapies. Tuberculosis, 91, 67–70.
Sethuraman, G., & Ramesh, V. (2013). Cutaneous tuberculosis in children. Pediatric Dermatology, 30, 7–16.
Sharma, A., Sharma, S., & Khuller, G. K. (2004). Lectin-functionalized poly (lactide-coglycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. Journal of Antimicrobial Chemotherapy, 54, 761–766.
Sharma, K., Somavarapu, S., Colombani, A., Govind, N., & Taylor, K. M. (2012). Crosslinked chitosan nanoparticle formulations for delivery from pressurized metered dose inhalers. European Journal of Pharmaceutics and Biopharmaceutics, 81, 74–81.
Shen, Z. G., Chen, W. H., Jugade, N., Gao, L. Y., Glover, W., Shen, J. Y., et al. (2012). Fabrication of inhalable spore like pharmaceutical particles for deep lung deposition. International Journal of Pharmaceutics, 430, 98–103.
Shingnapurkar, D., Dandawate, P., Anson, C. E., Powell, A. K., Afrasiabi, Z., Sinn, E., et al. (2012). Synthesis and characterization of pyruvate-isoniazid analogs and their copper complexes as potential ICL inhibitors. Bioorganic & Medicinal Chemistry Letters, 22, 3172–3176.
Siddiqi, K., Lambert, M. L., & Walley, J. (2003). Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: The current evidence. The Lancet Infectious Diseases, 3, 288–296.
Singh, H., Bhandari, R., & Kaur, I. P. (2013). Encapsulation of rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with isoniazid at acidic pH. International Journal of Pharmaceutics, 446, 106–111.
Son, Y. J., & McConville, J. T. (2011). A new respirable form of rifampicin. European Journal of Pharmaceutics and Biopharmaceutics, 78, 366–376.
Song, X., Lin, Q., Guo, L., Fu, Y., Han, J., & Ke, H. (2015). Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage specific delivery. Pharmaceutical Research, 32, 1741–1751.
Soo, P. C., Horng, Y. T., Chang, K. C., Wang, J. Y., Hsueh, P. R., Chuang, C. Y., et al. (2009). A simple gold nanoparticle probes assay for identification of Mycobacterium tuberculosis and Mycobacterium tuberculosis complex from clinical specimens. Molecular and Cellular Probes, 23, 240–246.
Sosnik, A., Carcaboso, A. M., Glisoni, R. J., Moretton, M. A., & Chiappetta, D. A. (2010). New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Advanced Drug Delivery Reviews, 62, 547–559.
Sung, J. C., Padilla, D. J., Garcia-Contreras, L., Verberkmoes, J. L., Durbin, D., Peloquin, C. A., et al. (2009). Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharmaceutical Research, 26, 1847–1855.
Sung, J. C., Pulliam, B. L., & Edwards, D. A. (2007). Nanoparticles for drug delivery to the lungs. Trends in Biotechnology, 25, 563–570.
Thanyani, S. T., Roberts, V., Siko, D. G. R., Vrey, P., & Verschoor, J. A. (2008). A novel application of affinity biosensor technology to detect antibodies to mycolic acid in tuberculosis patients. Journal of Immunological Methods, 332, 61–72.
Thiruppathiraja, C., Kamatchiammal, S., Adaikkappan, P., Santhosh, D. J., & Alagar, M. (2011). Specific detection of Mycobacterium sp. genomic DNA using dual labeled gold nanoparticle based electrochemical biosensor. Analytical Biochemistry, 417, 73–79.
Tom, R. T., Suryanarayanan, V., Reddy, P. G., Baskaran, S., & Pradeep, T. (2004). Ciprofloxacinprotected gold nanoparticles. Langmuir, 20, 1909–1914.
Turner, P. V., Brabb, T., Pekow, C., & Vasbinder, M. A. (2011). Administration of substances to laboratory animals: Routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science, 50, 600–613.
Van Rie, A., Page-Shipp, L., Scott, L., Sanne, I., & Stevens, W. (2010). Xpert® MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: Hype or hope? Expert Review of Molecular Diagnostics, 10, 937–946.
van Zyl, L., du Plessis, J., & Viljoen, J. (2015). Cutaneous tuberculosis overview and current treatment regimens. Tuberculosis, 95, 629–638.
Varma, J. N. R., Kumar, T. S., Prasanthi, B., & Ratna, J. V. (2015). Formulation and characterization of pyrazinamide polymeric nanoparticles for pulmonary tuberculosis: Efficiency for alveolar macrophage targeting. Indian Journal of Pharmaceutical Sciences, 77, 258–266.
Vashist, A., Vashist, A., Gupta, Y. K., & Ahmad, S. (2014). Recent advances in hydrogel based drug delivery systems for the human body. Journal of Materials Chemistry B, 2, 147–166.
Videira, M. A., Botelho, M. F., Santos, A. C., Gouveia, L. F., de Lima, J. J., & Almeida, A. J. (2002). Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. Journal of Drug Targeting, 10, 607–613.
Vyas, S. P., Kannan, M. E., Jain, S., Mishra, V., & Singh, P. (2004). Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. International Journal of Pharmaceutics, 269, 37–49.
Wallis, R. S., & Hafner, R. (2015). Advancing host-directed therapy for tuberculosis. Nature Reviews Immunology, 15, 255–263.
Wang, S., Xu, F., & Demirci, U. (2010). Advances in develo** HIV-1 viral load assays for resource-limited settings. Biotechnology Advances, 28, 770–781.
WHO. (2011). Global tuberculosis control. Retrieved Jan 26, 2012 from http://www.who.int/tb/publications/global_report/en/2011.
WHO. (2015a). Global tuberculosis report 2015. WHO Library Cataloguing-in-Publication Data.
WHO. (2015b). The use of delamanid in the treatment of multidrug-resistant tuberculosis. WHO Library Cataloguing-in-Publication Data.
Wijagkanalan, W., Kawakami, S., Takenaga, M., Igarashi, R., Yamashita, F., & Hashida, M. (2008). Efficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in rats. Journal of Controlled Release, 125, 121–130.
Willis, L., Hayes, D., Jr., & Mansour, H. M. (2012). Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery. Lung, 190, 251–262.
**e, H., Mire, J., Kong, Y., Chang, M., Hassounah, H. A., Thornton, C. N., et al. (2012). Rapid point-of-care detection of the tuberculosis pathogen using a BlaC-specific fluorogenic probe. Nature Chemistry, 4, 802–809.
Yadav, A. B., Singh, A. K., Verma, R. K., Mohan, M., Agrawal, A. K., & Misra, A. (2011). The devil’s advocacy: When and why inhaled therapies for tuberculosis may not work. Tuberculosis, 91, 65–66.
Yeo, W. H., Liu, S., Chung, J. H., Liu, Y. L., & Lee, K. H. (2009). Rapid detection of Mycobacterium tuberculosis cells by using microtip-based immunoassay. Analytical and Bioanalytical Chemistry, 393, 1593–1600.
Yu, W., Liu, C., Liu, Y., Zhang, N., & Xu, W. (2010). Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharmaceutical Research, 27, 1584–1596.
Zumla, A., Chakaya, J., Centis, R., D’Ambrosio, L., Mwaba, P., Bates, M., et al. (2015). Tuberculosis treatment and management—An update on treatment regimens, trials, new drugs, and adjunct therapies. The Lancet Respiratory Medicine, 3, 220–234.
Zwerling, A., Behr, M. A., Verma, A., Brewer, T. F., Menzies, D., & Pai, M. (2011). The BCG World Atlas: A database of global BCG vaccination policies and practices. PLoS Medicine, 8, e1001012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dakkah, A.N., Bataineh, Y., Jaidi, B.A.A., Bayan, M.F., Nimer, N.A. (2020). Nanomedicines in Tuberculosis: Diagnosis, Therapy and Nanodrug Delivery. In: Krishnan, A., Chuturgoon, A. (eds) Integrative Nanomedicine for New Therapies. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-36260-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-36260-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36259-1
Online ISBN: 978-3-030-36260-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)