Abstract
Ionic liquids satisfy the requirements of a gas chromatography stationary phase , among which characteristics include high viscosity, tunable selectivity through structural modifications, good wettability with respect to fused silica capillaries, and high thermal stability . When incorporated in either the first or second dimension of multidimensional gas chromatography, they offer unique selectivity compared to conventional gas chromatography stationary phases . The utilization of commercial ionic liquid columns for analysis of various analytes (i.e., fatty acids , flavors and fragrances , organic pollutants, and petrochemical samples) in complex matrices is described in this chapter. Thanks to their dual-nature behavior, IL-based stationary phases provide unique separation for both polar and nonpolar molecules in complex samples, such as essential oils . Moderately polar phosphonium-based ionic liquid columns (i.e., SLB-IL59, SLB-IL60, and SLB-IL61) with high operating temperature are suitable for analysis of complex petrochemical and environmental samples. The high polarity of ionic liquid columns allows analysis of positional isomers including those of unsaturated fatty acids . The other exceptional feature of ionic liquid columns is their stability in the presence of water and oxygen at high temperatures. Water-compatible ionic liquid -based gas chromatography capillary columns facilitate the direct injection of aqueous samples without the requirement of time-consuming sample pretreatment techniques.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Weingärtner H (2008) Understanding ionic liquids at the molecular level: facts, problems, and controversies. Angew Chem Int Ed 47:654–670. https://doi.org/10.1002/anie.200604951
Soukup-Hein RJ, Warnke MM, Armstrong DW (2009) Ionic liquids in analytical chemistry. Ann Rev Anal Chem 2:145–168. https://doi.org/10.1146/annurev-anchem-060908-155150
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99:2071–2083. https://doi.org/10.1021/cr980032t
Armstrong DW, He L, Liu Y-S (1999) Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal Chem 71:3873–3876. https://doi.org/10.1021/ac990443p
Anderson JL, Armstrong DW (2003) High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal Chem 75:4851–4858. https://doi.org/10.1021/ac0345749
Anderson JL, Armstrong DW (2005) Immobilized ionic liquids as high-selectivity/high-temperature/high-stability gas chromatography stationary phases. Anal Chem 77:6453–6462. https://doi.org/10.1021/ac051006f
Huang K, Han X, Zhang X, Armstrong DW (2007) PEG-linked geminal dicationic ionic liquids as selective, high-stability gas chromatographic stationary phases. Anal Bioanal Chem 389:2265–2275. https://doi.org/10.1007/s00216-007-1625-0
Breitbach ZS, Armstrong DW (2008) Characterization of phosphonium ionic liquids through a linear solvation energy relationship and their use as GLC stationary phases. Anal Bioanal Chem 390:1605–1617. https://doi.org/10.1007/s00216-008-1877-3
Payagala T, Zhang Y, Wanigasekara E, Huang K, Breitbach ZS, Sharma PS, Sidisky LM, Armstrong DW (2008) Trigonal tricationic ionic liquids: a generation of gas chromatographic stationary phases. Anal Chem 81:160–173. https://doi.org/10.1021/ac8016949
Barber DW, Phillips CSG, Tusa GF, Verdin A (1959) The chromatography of gases and vapours. Part VI. Use of the stearates of bivalent manganese, cobalt, nickel, copper, and zinc as column liquids in gas chromatography. J Chem Soc 18–24. https://doi.org/10.1039/JR9590000018
Pacholec F, Butler HT, Poole CF (1982) Molten organic salt phase for gas–liquid chromatography. Anal Chem 54:1938–1941. https://doi.org/10.1021/ac00249a006
Pacholec F, Poole C (1983) Stationary phase properties of the organic molten salt ethylpyridinium bromide in gas chromatography. Chromatographia 17:370–374. https://doi.org/10.1007/BF02262375
Pomaville RM, Poole SK, Davis LJ, Poole CF (1988) Solute—solvent interactions in tetra-n-butylphosphonium salts studied by gas chromatography. J Chromatogr A 438:1–14. https://doi.org/10.1016/S0021-9673(00)90227-9
Gordon JE, Selwyn JE, Thorne RL (1966) Molten quaternary ammonium salts as stationary liquid phases for gas–liquid partition chromatography. J Org Chem 31:1925–1930. https://doi.org/10.1021/jo01344a059
Poole CF, Butler HT, Coddens ME, Dhanesar SC, Pacholec F (1984) Survey of organic molten salt phases for gas chromatography. J Chromatogr A 289:299–320. https://doi.org/10.1016/S0021-9673(00)95096-9
Anderson JL, Ding J, Welton T, Armstrong DW (2002) Characterizing ionic liquids on the basis of multiple solvation interactions. J Am Chem Soc 124:14247–14254. https://doi.org/10.1021/ja028156h
Anderson JL, Ding R, Ellern A, Armstrong DW (2005) Structure and properties of high stability geminal dicationic ionic liquids. J Am Chem Soc 127:593–604. https://doi.org/10.1021/ja046521u
Patil RA, Talebi M, Sidisky LM, Armstrong DW (2017) Examination of selectivities of thermally stable geminal dicationic ionic liquids by structural modification. Chromatographia 80:1563–1574. https://doi.org/10.1007/s10337-017-3372-5
Patil RA, Talebi M, Sidisky LM, Berthod A, Armstrong DW (2018) Gas chromatography selectivity of new phosphonium-based dicationic ionic liquid stationary phases. J Sep Sci 41:4142–4148. https://doi.org/10.1002/jssc.201800695
Patil RA, Talebi M, Xu C, Bhawal SS, Armstrong DW (2016) Synthesis of thermally stable geminal dicationic ionic liquids and related ionic compounds: an examination of physicochemical properties by structural modification. Chem Mater 28:4315–4323. https://doi.org/10.1021/acs.chemmater.6b01247
Talebi M, Patil RA, Armstrong DW (2018) Physicochemical properties of branched-chain dicationic ionic liquids. J Mol Liq 256:247–255. https://doi.org/10.1016/j.molliq.2018.02.016
Sun P, Armstrong DW (2010) Ionic liquids in analytical chemistry. Anal Chim Acta 661:1–16. https://doi.org/10.1016/j.aca.2009.12.007
Talebi M, Patil RA, Sidisky LM, Berthod A, Armstrong DW (2018) Variation of anionic moieties of dicationic ionic liquid GC stationary phases: effect on stability and selectivity. Anal Chim Acta 1042:155–164. https://doi.org/10.1016/j.aca.2018.07.047
Frink LA, Armstrong DW (2016) Determination of trace water content in petroleum and petroleum products. Anal Chem 88:8194–8201. https://doi.org/10.1021/acs.analchem.6b02006
Frink LA, Armstrong DW (2016) Water determination in solid pharmaceutical products utilizing ionic liquids and headspace gas chromatography. J Pharm Sci 105:2288–2292. https://doi.org/10.1016/j.xphs.2016.05.014
Talebi M, Frink LA, Patil RA, Armstrong DW (2017) Examination of the varied and changing ethanol content of commercial Kombucha products. Food Anal Methods 10:4062–4067. https://doi.org/10.1007/s12161-017-0980-5
Payagala T, Huang J, Breitbach ZS, Sharma PS, Armstrong DW (2007) Unsymmetrical dicationic ionic liquids: manipulation of physicochemical properties using specific structural architectures. Chem Mater 19:5848–5850. https://doi.org/10.1021/cm702325a
Zeng Z, Phillips BS, **ao J-C, Shreeve JM (2008) Polyfluoroalkyl, polyethylene glycol, 1,4-bismethylenebenzene, or 1,4-bismethylene-2,3,5,6-tetrafluorobenzene bridged functionalized dicationic ionic liquids: synthesis and properties as high temperature lubricants. Chem Mater 20:2719–2726. https://doi.org/10.1021/cm703693r
Talebi M, Patil RA, Sidisky LM, Berthod A, Armstrong DW (2018) Branched-chain dicationic ionic liquids for fatty acid methyl ester assessment by gas chromatography. Anal Bioanal Chem 410:4633–4643. https://doi.org/10.1007/s00216-017-0722-y
McReynolds WO (1970) Characterization of some liquid phases. J Chromatogr Sci 8:685–691. https://doi.org/10.1093/chromsci/8.12.685; ibid. (1971) 9:15A. https://doi.org/10.1093/chromsci/9.5.15A-a.
Petsch M, Mayer-Helm BX, Söllner V (2005) Preparation and characterization of fused-silica capillary columns coated with m-carborane–siloxane copolymers for gas chromatography. Anal Bioanal Chem 383:322–326. https://doi.org/10.1007/s00216-005-3399-6
Ragonese C, Sciarrone D, Tranchida PQ, Dugo P, Dugo G, Mondello L (2011) Evaluation of a medium-polarity ionic liquid stationary phase in the analysis of flavor and fragrance compounds. Anal Chem 83:7947–7954. https://doi.org/10.1021/ac202012u
Abraham MH (1993) Scales of solute hydrogen-bonding: their construction and application to physicochemical and biochemical processes. Chem Soc Rev 22:73–83. https://doi.org/10.1039/CS9932200073
Abraham MH, Ibrahim A, Zissimos AM (2004) Determination of sets of solute descriptors from chromatographic measurements. J Chromatogr A 1037:29–47. https://doi.org/10.1016/j.chroma.2003.12.004
Abraham MH, Poole CF, Poole SK (1999) Classification of stationary phases and other materials by gas chromatography. J Chromatogr A 842:79–114. https://doi.org/10.1016/S0021-9673(98)00930-3
Weber W, Andersson JT (2014) Ionic liquids as stationary phases in gas chromatography—an LSER investigation of six commercial phases and some applications. Anal Bioanal Chem 406:5347–5358. https://doi.org/10.1007/s00216-014-7972-8
Grob Jr K, Grob G, Grob K (1978) Comprehensive, standardized quality test for glass capillary columns. J Chromatogr A 156:1–20. https://doi.org/10.1016/S0021-9673(00)83120-9
Grob K, Grob G, Grob Jr K (1981) Testing capillary gas chromatographic columns. J Chromatogr A 219:13–20. https://doi.org/10.1016/S0021-9673(00)80568-3
Dawczynski C, Martin L, Wagner A, Jahreis G (2010) n − 3 LC-PUFA-enriched dairy products are able to reduce cardiovascular risk factors: a double-blind, cross-over study. Clin Nutr 29:592–599. https://doi.org/10.1016/j.clnu.2010.02.008
Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M, Grün M, Nicolaou A, Jahreis G (2013) Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin Nutr 32:686–696. https://doi.org/10.1016/j.clnu.2012.12.010
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids. Elsevier, Amsterdam
Delmonte P, Fardin Kia A-R, Kramer JKG, Mossoba MM, Sidisky L, Rader JI (2011) Separation characteristics of fatty acid methyl esters using SLB-IL111, a new ionic liquid coated capillary gas chromatographic column. J Chromatogr A 1218:545–554. https://doi.org/10.1016/j.chroma.2010.11.072
Delmonte P, Fardin-Kia AR, Kramer JKG, Mossoba MM, Sidisky L, Tyburczy C, Rader JI (2012) Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J Chromatogr A 1233:137–146. https://doi.org/10.1016/j.chroma.2012.02.012
Zeng AX, Chin S-T, Nolvachai Y, Kulsing C, Sidisky LM, Marriott PJ (2013) Characterisation of capillary ionic liquid columns for gas chromatography–mass spectrometry analysis of fatty acid methyl esters. Anal Chim Acta 803:166–173. https://doi.org/10.1016/j.aca.2013.07.002
Delmonte P, Kia A-RF, Hu Q, Rader JI (2009) Review of methods for preparation and gas chromatographic separation of trans and cis reference fatty acids. J AOAC Int 92:1310–1326. https://www.ingentaconnect.com/content/aoac/jaoac/2009/00000092/00000005/art00007f
Tyburczy C, Delmonte P, Fardin-Kia AR, Mossoba MM, Kramer JKG, Rader JI (2012) Profile of trans fatty acids (FAs) including trans polyunsaturated FAs in representative fast food samples. J Agric Food Chem 60:4567–4577. https://doi.org/10.1021/jf300585s
Lin C-C, Wasta Z, Mjøs SA (2014) Evaluation of the retention pattern on ionic liquid columns for gas chromatographic analyses of fatty acid methyl esters. J Chromatogr A 1350:83–91. https://doi.org/10.1016/j.chroma.2014.05.023
Firestone D (2009) Official methods and recommended practices of the AOCS. In: American Oil Chemists' Society. http://agris.fao.org/agris-search/search.do?recordID=US201300136250
Precht D, Molkentin J (2000) Identification and quantitation of cis/trans C16:1 and C17:1 fatty acid positional isomers in German human milk lipids by thin-layer chromatography and gas chromatography/mass spectrometry. Eur J Lipid Sci Technol 102:102–113. https://doi.org/10.1002/(SICI)1438-9312(200002)102:2%3C102::AID-EJLT102%3E3.0.CO;2-C
Precht D, Molkentin J (1996) Rapid analysis of the isomers of trans-octadecenoic acid in milk fat. Int Dairy J 6:791–809. https://doi.org/10.1016/0958-6946(96)00004-0
Sehat N, Rickert R, Mossoba MM, Kramer JKG, Yurawecz MP, Roach JAG, Adlof RO, Morehouse KM, Fritsche J, Eulitz KD, Steinhart H, Ku Y (1999) Improved separation of conjugated fatty acid methyl esters by silver ion–high-performance liquid chromatography. Lipids 34:407–413. https://doi.org/10.1007/s11745-999-0379-3
Yurawecz MP, Roach JAG, Sehat N, Mossoba MM, Kramer JKG, Fritsche J, Steinhart H, Ku Y (1998) A new conjugated linoleic acid isomer, 7 trans, 9 cis-octadecadienoic acid, in cow milk, cheese, beef and human milk and adipose tissue. Lipids 33:803–809. https://doi.org/10.1007/s11745-998-0273-z
Fanali C, Micalizzi G, Dugo P, Mondello L (2017) Ionic liquids as stationary phases for fatty acid analysis by gas chromatography. Analyst 142:4601–4612. https://doi.org/10.1039/C7AN01338H
Shibamoto S, Gooley A, Yamamoto K (2015) Separation behavior of octadecadienoic acid isomers and identification of cis- and trans-isomers using gas chromatography. Lipids 50:85–100. https://doi.org/10.1007/s11745-014-3966-8
Delmonte P (2016) Evaluation of poly(90% biscyanopropyl/10% cyanopropylphenyl siloxane) capillary columns for the gas chromatographic quantification of trans fatty acids in non-hydrogenated vegetable oils. J Chromatogr A 1460:160–172. https://doi.org/10.1016/j.chroma.2016.07.019
Granafei S, Losito I, Salivo S, Tranchida PQ, Mondello L, Palmisano F, Cataldi TRI (2015) Occurrence of oleic and 18:1 methyl-branched acyl chains in lipids of Rhodobacter sphaeroides 2.4.1. Anal Chim Acta 885:191–198. https://doi.org/10.1016/j.aca.2015.05.028
Ragonese C, Tranchida PQ, Dugo P, Dugo G, Sidisky LM, Robillard MV, Mondello L (2009) Evaluation of use of a dicationic liquid stationary phase in the fast and conventional gas chromatographic analysis of health-hazardous C18 cis/trans fatty acids. Anal Chem 81:5561–5568. https://doi.org/10.1021/ac9007094
Delmonte P, Fardin-Kia AR, Rader JI (2013) Separation of fatty acid methyl esters by GC-online hydrogenation × GC. Anal Chem 85:1517–1524. https://doi.org/10.1021/ac302707z
Fardin-Kia AR, Delmonte P, Kramer JKG, Jahreis G, Kuhnt K, Santercole V, Rader JI (2013) Separation of the fatty acids in menhaden oil as methyl esters with a highly polar ionic liquid gas chromatographic column and identification by time of flight mass spectrometry. Lipids 48:1279–1295. https://doi.org/10.1007/s11745-013-3830-2
Dettmer K (2014) Assessment of ionic liquid stationary phases for the GC analysis of fatty acid methyl esters. Anal Bioanal Chem 406:4931–4939. https://doi.org/10.1007/s00216-014-7919-0
Mjøs SA (2003) Identification of fatty acids in gas chromatography by application of different temperature and pressure programs on a single capillary column. J Chromatogr A 1015:151–161. https://doi.org/10.1016/S0021-9673(03)01240-8
Mjøs SA, Grahl-Nielsen O (2006) Prediction of gas chromatographic retention of polyunsaturated fatty acid methyl esters. J Chromatogr A 1110:171–180. https://doi.org/10.1016/j.chroma.2006.01.092
Pinto AC, Guarieiro LLN, Rezende MJC, Ribeiro NM, Torres EA, Lopes WA, de P Pereira PA, de Andrade JB (2005) Biodiesel: an overview. J Braz Chem Soc 16:1313–1330. http://dx.doi.org/10.1590/S0103-50532005000800003
Webster RL, Rawson PM, Evans DJ, Marriott PJ (2016) Quantification of trace fatty acid methyl esters in diesel fuel by using multidimensional gas chromatography with electron and chemical ionization mass spectrometry. J Sep Sci 39:2537–2543. https://doi.org/10.1002/jssc.201600307
Ragonese C, Tranchida PQ, Sciarrone D, Mondello L (2009) Conventional and fast gas chromatography analysis of biodiesel blends using an ionic liquid stationary phase. J Chromatogr A 1216:8992–8997. https://doi.org/10.1016/j.chroma.2009.10.066
Goding JC, Ragon DY, O’Connor JB, Boehm SJ, Hupp AM (2013) Comparison of GC stationary phases for the separation of fatty acid methyl esters in biodiesel fuels. Anal Bioanal Chem 405:6087–6094. https://doi.org/10.1007/s00216-013-7042-7
Mogollon NGS, de Lima Ribeiro FA, Lopez MM, Hantao LW, Poppi RJ, Augusto F (2013) Quantitative analysis of biodiesel in blends of biodiesel and conventional diesel by comprehensive two-dimensional gas chromatography and multivariate curve resolution. Anal Chim Acta 796:130–136. https://doi.org/10.1016/j.aca.2013.07.071
Takahashi Sato R, Stroppa PHF, da Silva AD, de Oliveira MAL (2016) Fast GC-FID method for monitoring acidic and basic catalytic transesterification reactions in vegetable oils to methyl ester biodiesel preparation. Quim Nova 39:352–355. http://dx.doi.org/10.5935/0100-4042.20160027
Mogollón NGS, Ribeiro FAL, Poppi RJ, Quintana AL, Chávez JAG, Agualongo DAP, Aleme HG, Augusto F (2017) Exploratory analysis of biodiesel by combining comprehensive two-dimensional gas chromatography and multiway principal component analysis. J Braz Chem Soc 28:740–746. http://dx.doi.org/10.21577/0103-5053.20160222
Webster RL, Evans DJ, Marriott PJ (2015) Detailed chemical analysis using multidimensional gas chromatography–mass spectrometry and bulk properties of low-temperature oxidized jet fuels. Energy Fuels 29:2059–2066. https://doi.org/10.1021/acs.energyfuels.5b00264
McCormick RL, Graboski MS, Alleman TL, Herring AM, Tyson KS (2001) Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environ Sci Technol 35:1742–1747. https://doi.org/10.1021/es001636t
Sushchik NN, Kuchkina AYu, Gladyshev MI (2013) Fatty acid content and composition of sediments from Siberian eutrophic water bodies: Implications for biodiesel production. Water Res 47:3192–3200. https://doi.org/10.1016/j.watres.2013.03.031
Knothe G, Sharp CA, Ryan III TW (2006) Exhaust emissions of biodiesel, petrodiesel, neat methyl esters, and alkanes in a new technology engine. Energy Fuels 20:403–408. https://doi.org/10.1021/ef0502711
CSN EN 14331 (2004) Liquid petroleum products - Separation and characterization of fatty acid methyl esters (FAME) from middle distillates - Liquid chromatography (LC)/gas chromatography (GC). European Committee for Standardization, Brussels
Katona G, Andréasson U, Landau EM, Andréasson L-E, Neutze R (2003) Lipidic cubic phase crystal structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.35 Å resolution. J Mol Biol 331:681–692. https://doi.org/10.1016/S0022-2836(03)00751-4
Weatherly CA, Zhang Y, Smuts JP, Fan H, Xu C, Schug KA, Lang JC, Armstrong DW (2016) Analysis of long-chain unsaturated fatty acids by ionic liquid gas chromatography. J Agric Food Chem 64:1422–1432. https://doi.org/10.1021/acs.jafc.5b05988
Tyburczy C, Mossoba MM, Rader JI (2013) Determination of trans fat in edible oils: current official methods and overview of recent developments. Anal Bioanal Chem 405:5759–5772. https://doi.org/10.1007/s00216-013-7005-z
Verméglio A, Joliot P (1999) The photosynthetic apparatus of Rhodobacter sphaeroides. Trends Microbiol 7:435–440. https://doi.org/10.1016/S0966-842X(99)01625-X
Zeng X, Roh JH, Callister SJ, Tavano CL, Donohue TJ, Lipton MS, Kaplan S (2007) Proteomic characterization of the Rhodobacter sphaeroides 2.4.1 photosynthetic membrane: identification of new proteins. J Bacteriol 189:7464–7474. https://doi.org/10.1128/JB.00946-07
Tucker JD, Siebert CA, Escalante M, Adams PG, Olsen JD, Otto C, Stokes DL, Hunter CN (2010) Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Mol Microbiol 76:833–847. https://doi.org/10.1111/j.1365-2958.2010.07153.x
Destaillats F, Guitard M, Cruz-Hernandez C (2011) Identification of Δ6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography–mass-spectrometry using ionic-liquid coated capillary column. J Chromatogr A 1218:9384–9389. https://doi.org/10.1016/j.chroma.2011.10.095
Inagaki S, Numata M (2015) Fast GC analysis of fatty acid methyl esters using a highly polar ionic liquid column and its application for the determination of trans fatty acid contents in edible oils. Chromatographia 78:291–295. https://doi.org/10.1007/s10337-014-2837-z
Yoshinaga K, Asanuma M, Mizobe H, Kojima K, Nagai T, Beppu F, Gotoh N (2014) Characterization of cis- and trans-octadecenoic acid positional isomers in edible fat and oil using gas chromatography–flame ionisation detector equipped with highly polar ionic liquid capillary column. Food Chem 160:39–45. https://doi.org/10.1016/j.foodchem.2014.03.069
Lock AL, Bauman DE (2004) Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 39:1197–1206. https://doi.org/10.1007/s11745-004-1348-6
Aldai N, Dugan MER, Rolland DC, Kramer JKG (2009) Survey of the fatty acid composition of Canadian beef: backfat and longissimus lumborum muscle. Can J Anim Sci 89:315–329. https://doi.org/10.4141/CJAS08126
Cruz-Hernandez C, Kramer JKG, Kennelly JJ, Glimm DR, Sorensen BM, Okine EK, Goonewardene LA, Weselake RJ (2007) Evaluating the conjugated linoleic acid and trans 18:1 isomers in milk fat of dairy cows fed increasing amounts of sunflower oil and a constant level of fish oil. J Dairy Sci 90:3786–3801. https://doi.org/10.3168/jds.2006-698
Bauman DE, Griinari JM (2003) Nutritional regulation of milk fat synthesis. Annu Rev Nutr 23:203–227. https://doi.org/10.1146/annurev.nutr.23.011702.073408
Gómez-Cortés P, Rodríguez-Pino V, Juárez M, de la Fuente MA (2017) Optimization of milk odd and branched-chain fatty acids analysis by gas chromatography using an extremely polar stationary phase. Food Chem 231:11–18. https://doi.org/10.1016/j.foodchem.2017.03.052
Fievez V, Colman E, Castro-Montoya JM, Stefanov I, Vlaeminck B (2012) Milk odd- and branched-chain fatty acids as biomarkers of rumen function—an update. Anim Feed Sci Technol 172:51–65. https://doi.org/10.1016/j.anifeedsci.2011.12.008
Tyburczy Srigley C, Rader JI (2014) Content and composition of fatty acids in marine oil omega-3 supplements. J Agric Food Chem 62:7268–7278. https://doi.org/10.1021/jf5016973
Ando Y, Sasaki T (2011) GC separation of cis-eicosenoic acid positional isomers on an ionic liquid SLB-IL100 stationary phase. J Am Oil Chem Soc 88:743–748. https://doi.org/10.1007/s11746-010-1733-4
Hammann S, Vetter W (2015) Gas chromatographic separation of fatty acid esters of cholesterol and phytosterols on an ionic liquid capillary column. J Chromatogr B 1007:67–71. https://doi.org/10.1016/j.jchromb.2015.11.007
Moreau RA, Whitaker BD, Hicks KB (2002) Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41:457–500. https://doi.org/10.1016/S0163-7827(02)00006-1
Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH (2002) Free and esterified sterol composition of edible oils and fats. J Food Compos Anal 15:123–142. https://doi.org/10.1006/jfca.2001.1044
Evershed RP, Male VL, Goad LJ (1987) Strategy for the analysis of steryl esters from plant and animal tissues. J Chromatogr A 400:187–205. https://doi.org/10.1016/S0021-9673(01)81612-5
Fan H, Smuts J, Bai L, Walsh P, Armstrong DW, Schug KA (2016) Gas chromatography–vacuum ultraviolet spectroscopy for analysis of fatty acid methyl esters. Food Chem 194:265–271. https://doi.org/10.1016/j.foodchem.2015.08.004
Nan H, Anderson JL (2018) Ionic liquid stationary phases for multidimensional gas chromatography. Trends Anal Chem 105:367–379. https://doi.org/10.1016/j.trac.2018.03.020
Nolvachai Y, Kulsing C, Marriott PJ (2015) Thermally sensitive behavior explanation for unusual orthogonality observed in comprehensive two-dimensional gas chromatography comprising a single ionic liquid stationary phase. Anal Chem 87:538–544. https://doi.org/10.1021/ac5030039
Gu Q, David F, Lynen F, Vanormelingen P, Vyverman W, Rumpel K, Xu G, Sandra P (2011) Evaluation of ionic liquid stationary phases for one dimensional gas chromatography–mass spectrometry and comprehensive two dimensional gas chromatographic analyses of fatty acids in marine biota. J Chromatogr A 1218:3056–3063. https://doi.org/10.1016/j.chroma.2011.03.011
Nosheen A, Mitrevski B, Bano A, Marriott PJ (2013) Fast comprehensive two-dimensional gas chromatography method for fatty acid methyl ester separation and quantification using dual ionic liquid columns. J Chromatogr A 1312:118–123. https://doi.org/10.1016/j.chroma.2013.08.099
Zeng AX, Chin S-T, Marriott PJ (2013) Integrated multidimensional and comprehensive 2D GC analysis of fatty acid methyl esters. J Sep Sci 36:878–885. https://doi.org/10.1002/jssc.201200923
Pojjanapornpun S, Nolvachai Y, Aryusuk K, Kulsing C, Krisnangkura K, Marriott PJ (2018) Ionic liquid phases with comprehensive two-dimensional gas chromatography of fatty acid methyl esters. Anal Bioanal Chem 410:4669–4677. https://doi.org/10.1007/s00216-018-0944-7
Villegas C, Zhao Y, Curtis JM (2010) Two methods for the separation of monounsaturated octadecenoic acid isomers. J Chromatogr A 1217:775–784. https://doi.org/10.1016/j.chroma.2009.12.011
Qi M, Armstrong DW (2007) Dicationic ionic liquid stationary phase for GC-MS analysis of volatile compounds in herbal plants. Anal Bioanal Chem 388:889–899. https://doi.org/10.1007/s00216-007-1290-3
Cagliero C, Bicchi C, Cordero C, Liberto E, Sgorbini B, Rubiolo P (2012) Room temperature ionic liquids: New GC stationary phases with a novel selectivity for flavor and fragrance analyses. J Chromatogr A 1268:130–138. https://doi.org/10.1016/j.chroma.2012.10.016
Blumberg LM (2011) Metrics of separation performance in chromatography. Part 1. Definitions and application to static analyses. J Chromatogr A 1218:5375–5385. https://doi.org/10.1016/j.chroma.2011.06.017
Serôdio P, Nogueira JMF (2006) Considerations on ultra-trace analysis of phthalates in drinking water. Water Res 40:2572–2582. https://doi.org/10.1016/j.watres.2006.05.002
Polo M, Llompart M, Garcia-Jares C, Cela R (2005) Multivariate optimization of a solid-phase microextraction method for the analysis of phthalate esters in environmental waters. J Chromatogr A 1072:63–72. https://doi.org/10.1016/j.chroma.2004.12.040
Nassar N, Abeywardana P, Barker A, Bower C (2010) Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occup Environ Med 67:585–589. https://doi.org/10.1136/oem.2009.048272
Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM (2013) Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr 163:747–753.e1. https://doi.org/10.1016/j.jpeds.2013.03.072
Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP (2015) The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: the HOME study. Environ Health 14:75. https://doi.org/10.1186/s12940-015-0062-3
Jaakkola JJK, Knight TL (2008) The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect 116:845–853. https://doi.org/10.1289/ehp.10846
Net S, Delmont A, Sempéré R, Paluselli A, Ouddane B (2015) Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): a review. Sci Total Environ 515–516:162–180. https://doi.org/10.1016/j.scitotenv.2015.02.013
Li D, Stevens R, English C, GC-MS analysis of phthalates: comparison of GC stationary phase performance. https://www.restek.com/Technical-Resources/Technical-Library/General-Interest/gen_GNAN2380B-UNV, last time accessed: 19 Dec 2018
Sanchez-Prado L, Lamas JP, Garcia-Jares C, Llompart M (2012) Expanding the applications of the ionic liquids as GC stationary phases: plasticizers and synthetic musks fragrances. Chromatographia 75:1039–1047. https://doi.org/10.1007/s10337-012-2280-y
de Boer J, Blok D, Ballesteros-Gómez A (2014) Assessment of ionic liquid stationary phases for the determination of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers. J Chromatogr A 1348:158–163. https://doi.org/10.1016/j.chroma.2014.05.001
Kimbrough RD, Jensen AA (1989) Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products, Elsevier. Amsterdam. https://doi.org/10.1016/C2009-0-12642-9
Ballschmiter K, Bacher R, Mennel A, Fischer R, Riehle U, Swerev M (1992) The determination of chlorinated biphenyls, chlorinated dibenzodioxins, and chlorinated dibenzofurans by GC-MS. J High Resolut Chromatogr 15:260–270. https://doi.org/10.1002/jhrc.1240150411
Ros M, Escobar-Arnanz J, Sanz ML, Ramos L (2018) Evaluation of ionic liquid gas chromatography stationary phases for the separation of polychlorinated biphenyls. J Chromatogr A 1559:156–163. https://doi.org/10.1016/j.chroma.2017.12.029
Wilson WB, Sander LC, Oña-Ruales JO, Mössner SG, Sidisky LM, Lee ML, Wise SA (2017) Retention behavior of isomeric polycyclic aromatic sulfur heterocycles in gas chromatography on stationary phases of different selectivity. J Chromatogr A 1485:120–130. https://doi.org/10.1016/j.chroma.2017.01.024
Poster DL, Schantz MM, Sander LC, Wise SA (2006) Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatographic (GC) methods. Anal Bioanal Chem 386:859–881. https://doi.org/10.1007/s00216-006-0771-0
Zeigler C, Schantz M, Wise S, Robbat Jr A (2012) Mass spectra and retention indexes for polycyclic aromatic sulfur heterocycles and some alkylated analogs. Polycyc Aromatic Compds 32:154–176. https://doi.org/10.1080/10406638.2011.651679
Antle P, Zeigler C, Robbat Jr A (2014) Retention behavior of alkylated polycyclic aromatic sulfur heterocycles on immobilized ionic liquid stationary phases. J Chromatogr A 1361:255–264. https://doi.org/10.1016/j.chroma.2014.08.010
Krupčík J, Gorovenko R, Špánik I, Bočková I, Sandra P, Armstrong DW (2013) On the use of ionic liquid capillary columns for analysis of aromatic hydrocarbons in low-boiling petrochemical products by one-dimensional and comprehensive two-dimensional gas chromatography. J Chromatogr A 1301:225–236. https://doi.org/10.1016/j.chroma.2013.05.075
Nizio KD, Harynuk JJ (2012) Analysis of alkyl phosphates in petroleum samples by comprehensive two-dimensional gas chromatography with nitrogen phosphorus detection and post-column Deans switching. J Chromatogr A 1252:171–176. https://doi.org/10.1016/j.chroma.2012.06.070
Weber BM, Harynuk JJ (2013) Gas chromatographic retention of alkyl phosphates on ionic liquid stationary phases. J Chromatogr A 1271:170–175. https://doi.org/10.1016/j.chroma.2012.10.052
Dutriez T, Borras J, Courtiade M, Thiébaut D, Dulot H, Bertoncini F, Hennion M-C (2011) Challenge in the speciation of nitrogen-containing compounds in heavy petroleum fractions by high temperature comprehensive two-dimensional gas chromatography. J Chromatogr A 1218:3190–3199. https://doi.org/10.1016/j.chroma.2010.10.056
Mahé L, Dutriez T, Courtiade M, Thiébaut D, Dulot H, Bertoncini F (2011) Global approach for the selection of high temperature comprehensive two-dimensional gas chromatography experimental conditions and quantitative analysis in regards to sulfur-containing compounds in heavy petroleum cuts. J Chromatogr A 1218:534–544. https://doi.org/10.1016/j.chroma.2010.11.065
Cappelli Fontanive F, Souza-Silva ÉA, Macedo da Silva J, Bastos Caramão E, Alcaraz Zini C (2016) Characterization of sulfur and nitrogen compounds in Brazilian petroleum derivatives using ionic liquid capillary columns in comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection. J Chromatogr A 1461:131–143. https://doi.org/10.1016/j.chroma.2016.07.025
Padivitage NLT, Smuts JP, Armstrong DW (2014). Water determination. In: Riley CM, Rosanske TW, Rabel Riley SR (eds.) Specification of drug substances and products: development and validation of analytical methods. Elsevier, Oxford, pp 223–241. https://doi.org/10.1016/B978-0-08-098350-9.00011-4
Armstrong DW (2017) Measuring water: the expanding role of gas chromatography. LC GC 35:503–506. http://www.chromatographyonline.com/measuring-water-expanding-role-gas-chromatography
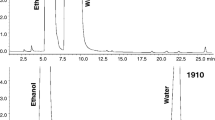
Weatherly CA, Woods RM, Armstrong DW (2014) Rapid analysis of ethanol and water in commercial products using ionic liquid capillary gas chromatography with thermal conductivity detection and/or barrier discharge ionization detection. J Agric Food Chem 62:1832–1838. https://doi.org/10.1021/jf4050167
Gallina A, Stocco N, Mutinelli F (2010) Karl Fischer titration to determine moisture in honey: a new simplified approach. Food Control 21:942–944. https://doi.org/10.1016/j.foodcont.2009.11.008
Isengard H-D, Präger H (2003) Water determination in products with high sugar content by infrared drying. Food Chem 82:161–167. https://doi.org/10.1016/S0308-8146(02)00538-1
Inagaki S, Morii N, Numata M (2015) Development of a reliable method to determine water content by headspace gas chromatography/mass spectrometry with the standard addition technique. Anal Methods 7:4816–4820. https://doi.org/10.1039/C5AY00832H
Hanna WS, Johnson AB (1950) Water content of hydrocarbons. Modified Karl Fischer method. Anal Chem 22:555–558. https://doi.org/10.1021/ac60040a012
Ivanova PG, Aneva ZV (2006) Assessment and assurance of quality in water measurement by coulometric Karl Fischer titration of petroleum products. Accred Qual Assur 10:543–549. https://doi.org/10.1007/s00769-005-0056-x
Margolis SA, Hagwood C (2003) The determination of water in crude oil and transformer oil reference materials. Anal Bioanal Chem 376:260–269. https://doi.org/10.1007/s00216-003-1865-6
Cedergren A, Nordmark U (2000) Determination of water in NIST reference material for mineral oils. Anal Chem 72:3392–3395. https://doi.org/10.1021/ac9913006
Larsson W, Jalbert J, Gilbert R, Cedergren A (2003) Efficiency of methods for Karl Fischer determination of water in oils based on oven evaporation and azeotropic distillation. Anal Chem 75:1227–1232. https://doi.org/10.1021/ac026229+
Frink LA, Weatherly CA, Armstrong DW (2014) Water determination in active pharmaceutical ingredients using ionic liquid headspace gas chromatography and two different detection protocols. J Pharm Biomed Anal 94:111–117. https://doi.org/10.1016/j.jpba.2014.01.034
Frink LA, Armstrong DW (2016) The utilisation of two detectors for the determination of water in honey using headspace gas chromatography. Food Chem 205:23–27. https://doi.org/10.1016/j.foodchem.2016.02.118
Frink LA, Armstrong DW (2017) Using headspace gas chromatography for the measurement of water in sugar and sugar-free sweeteners and products. LC GC 30:6–10. http://www.chromatographyonline.com/using-headspace-gas-chromatography-measurement-water-sugar-and-sugar-free-sweeteners-and-products-1
Xu B-Q, Rao C-Q, Cui S-F, Wang J, Wang J-L, Liu L-P (2018) Determination of trace water contents of organic solvents by gas chromatography-mass spectrometry-selected ion monitoring. J Chromatogr A 1570:109–115. https://doi.org/10.1016/j.chroma.2018.07.068
Zhang Y, Wang C, Armstrong DW, Woods RW, Jayawardhana DA (2011) Rapid, efficient quantification of water in solvents and solvents in water using an ionic liquid-based GC column. LC GC EUROPE 24:516–529
Knight HS, Weiss FT (1962) Determination of traces of water in hydrocarbons. A calcium carbide-gas liquid chromatography method. Anal Chem 34:749–751. https://doi.org/10.1021/ac60187a010
Quiram ER (1963) Applications of wide-diameter open tubular columns in gas chromatography. Anal Chem 35:593–595. https://doi.org/10.1021/ac60197a019
Kolb B, Auer M (1990) Analysis of water in liquid and solid samples by headspace gas chromatography. Part II: Insoluble solid samples by the “suspension approach”. Fresenius J Anal Chem 336:297–302. https://doi.org/10.1007/BF00331387
O’Keefe WK, Ng FTT, Rempel GL (2008) Validation of a gas chromatography/thermal conductivity detection method for the determination of the water content of oxygenated solvents. J Chromatogr A 1182:113–118. https://doi.org/10.1016/j.chroma.2007.12.044
Streim HG, Boyce EA, Smith JR (1961) Determination of water in 1,1-dimethylhydrazine, diethylenetriamine, and mixtures. Anal Chem 33:85–89. https://doi.org/10.1021/ac60169a025
Jayabalan R, Malbaša RV, Lončar ES, Vitas JS, Sathishkumar M (2014) A review on kombucha tea—microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Safety 13:538–550. https://doi.org/10.1111/1541-4337.12073
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Talebi, M., Patil, R.A., Armstrong, D.W. (2020). Gas Chromatography Columns Using Ionic Liquids as Stationary Phase. In: Shiflett, M. (eds) Commercial Applications of Ionic Liquids. Green Chemistry and Sustainable Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-35245-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-35245-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35244-8
Online ISBN: 978-3-030-35245-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)