Abstract
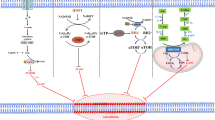
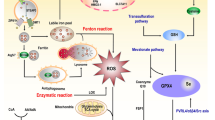
Ferroptosis, firstly demonstrated in 2012, is a type of iron- and lipid hydroperoxides-dependent regulated cell death. This newly recognized cell death morphologically, biochemically and genetically distinct from other types of cell death including apoptosis, necrosis, pyroptosis and autophagy. A series of strategies have been developed to induce ferroptosis to eliminate cancer cells, including overexpression or knockdown of ferroptosis-related genes, use of clinical drugs, chemical compounds, and iron-containing nanomaterials. A large number of studies have raised that ferroptosis might be a new option for clinical cancer therapy. However, it still exists long distance between the findings in the laboratory and the effective use in clinical cancer treatment using ferroptosis. Here, we introduced the main mechanism of ferroptosis and how potential ferrotposis is inhibited in different cancer types, and summarized the gene targets (GPX4, SLC7A11, ACSL4, CARS, SAT1, DPP4, NRF2, CD44v, CISD1, HSPB1), drug inducers (erastin and its analogs, RSL3 and its analogs, inhibitors of GSH synthesis, FINO2, statins, ART) and nanomaterial inducers (iron oxide nanoparticles, amorphous iron nanoparticles, iron–organic frameworks, iron-platinum nanoparticles and other indirect iron-based nanomaterials) for ferroptosis. With the advancement of ferroptosis theory, great progress in clinical cancer therapy might be achieved in the future.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abrams RP, Carroll WL, Woerpel KA (2016) Five-membered ring peroxide selectively initiates ferroptosis in cancer cells. ACS Chem Biol 11:1305–1312
Alvarez SW et al (2017) NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551:639–643
Baer MR, Augustinos P, Kinniburgh AJ (1992) Defective c-myc and c-myb RNA turnover in acute myeloid leukemia cells. Blood 79:1319–1326
Bannai S, Kitamura E (1980) Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 255:2372–2376
Brigelius-Flohe R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta 1830:3289–3303
Bruix J et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66
Chen Y, Zhang Z, Yang K, Du J, Xu Y, Liu S (2015) Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor growth through enforcing ferroportin-conducted iron egress. Oncogene 34:3839–3847
Cheng Z, Li Y (2007) What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem Rev 107:748–766
Cramer SL et al (2017) Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 23:120–127
DeNicola GM et al (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475:106–109
Dixon SJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072
Doll S et al (2017) ACSL4 dictates ferroptosis sensitivity by sha** cellular lipid composition. Nat Chem Biol 13:91–98
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3:285–296
Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR (2015) Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2:517–532
Fradejas N, Carlson BA, Rijntjes E, Becker NP, Tobe R, Schweizer U (2013) Mammalian Trit1 is a tRNA([Ser]Sec)-isopentenyl transferase required for full selenoprotein expression. Biochem J 450:427–432
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59:298–308
Gaschler MM et al (2018) FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14:507–515
Geldenhuys WJ, Leeper TC, Carroll RT (2014) mitoNEET as a novel drug target for mitochondrial dysfunction. Drug Discov Today 19:1601–1606
Guo J et al (2018) Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat 50:445–460
Hao S et al (2017) Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia 19:1022–1032
Hasegawa M et al (2016) Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 7:11756–11769
Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR (2016) Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ 23:270–278
Houessinon A et al (2016) Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol Cancer 15:38
Huo H, Zhou Z, Qin J, Liu W, Wang B, Gu Y (2016) Erastin disrupts mitochondrial permeability transition pore (mPTP) and induces apoptotic death of colorectal cancer cells. PLoS One 11:e0154605
Ishimoto T et al (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19:387–400
Jennis M et al (2016) An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev 30:918–930
Jiang L et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520:57–62
Joseph CA, Maroney MJ (2007) Cysteine dioxygenase: structure and mechanism. Chem Commun (Camb):3338–3349
Kennedy D et al (2017) HSPB1 facilitates ERK-mediated phosphorylation and degradation of BIM to attenuate endoplasmic reticulum stress-induced apoptosis. Cell Death Dis 8:e3026
Kim SE et al (2016) Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11:977–985
Komatsu M et al (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223
Kwon MY, Park E, Lee SJ, Chung SW (2015) Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6:24393–24403
Lachaier E et al (2014) Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res 34:6417–6422
Larraufie MH, Yang WS, Jiang E, Thomas AG, Slusher BS, Stockwell BR (2015) Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg Med Chem Lett 25:4787–4792
Li WP, Su CH, Chang YC, Lin YJ, Yeh CS (2016) Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome. ACS Nano 10:2017–2027
Lin CH et al (2016) Decreased mRNA expression for the two subunits of system xc(-), SLC3A2 and SLC7A11, in WBC in patients with schizophrenia: evidence in support of the hypo-glutamatergic hypothesis of schizophrenia. J Psychiatr Res 72:58–63
Lo M, Wang YZ, Gout PW (2008) The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 215:593–602
Ma S, Henson ES, Chen Y, Gibson SB (2016) Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis 7:e2307
Ma P et al (2017) Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett 17:928–937
Marengo B et al (2008) Mechanisms of BSO (L-buthionine-S,R-sulfoximine)-induced cytotoxic effects in neuroblastoma. Free Radic Biol Med 44:474–482
Ou Y, Wang SJ, Li D, Chu B, Gu W (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA 113:E6806–E6812
Schott C, Graab U, Cuvelier N, Hahn H, Fulda S (2015) Oncogenic RAS mutants confer resistance of RMS13 rhabdomyosarcoma cells to oxidative stress-induced ferroptotic cell death. Front Oncol 5:131
Sehm T et al (2016) Sulfasalazine impacts on ferroptotic cell death and alleviates the tumor microenvironment and glioma-induced brain edema. Oncotarget 7:36021–36033
Shen J et al (2014) Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics 4:487–497
Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X (2018) Emerging strategies of cancer therapy based on ferroptosis. Adv Mater 30:e1704007
Shimada K et al (2016) Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12:497–503
Shitara K et al (2017) Dose-escalation study for the targeting of CD44v(+) cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer 20:341–349
Sindrilaru A et al (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121:985–997
Skouta R et al (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136:4551–4556
Sohn YS et al (2013) NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc Natl Acad Sci USA 110:14676–14681
Stockwell BR et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285
Su YL, Fang JH, Liao CY, Lin CT, Li YT, Hu SH (2015) Targeted mesoporous iron oxide nanoparticles-encapsulated perfluorohexane and a hydrophobic drug for deep tumor penetration and therapy. Theranostics 5:1233–1248
Sun X et al (2015) HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34:5617–5625
Sun X et al (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63:173–184
Tan S, Schubert D, Maher P (2001) Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1:497–506
Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase. characterization of the enzyme. J Biol Chem 244:6388–6394
Viswanathan VS et al (2017) Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547:453–457
Wang SJ, Ou Y, Jiang L, Gu W (2016a) Ferroptosis: a missing puzzle piece in the p53 blueprint? Mol Cell Oncol 3:e1046581
Wang Y et al (2016b) In vivo MR and fluorescence dual-modality imaging of atherosclerosis characteristics in mice using profilin-1 targeted magnetic nanoparticles. Theranostics 6:272–286
Weiwer M et al (2012) Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg Med Chem Lett 22:1822–1826
Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE (2007) MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA 104:5318–5323
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11:441–469
Wu FQ et al (2016) ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. J Hepatol 65:314–324
**e Y et al (2017) The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 20:1692–1704
Yagoda N et al (2007) RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868
Yang WS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156:317–331
Yen SK, Padmanabhan P, Selvan ST (2013) Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics 3:986–1003
Yu Y et al (2015) The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2:e1054549
Yuan H, Li X, Zhang X, Kang R, Tang D (2016a) CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun 478:838–844
Yuan H, Li X, Zhang X, Kang R, Tang D (2016b) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 478:1338–1343
Yue L et al (2017) pH-responsive, self-sacrificial nanotheranostic agent for potential in vivo and in vitro dual modal MRI/CT imaging, real-time, and in situ monitoring of cancer therapy. Bioconjug Chem 28:400–409
Zanganeh S et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11:986–994
Zhang C et al (2016a) Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed Engl 55:2101–2106
Zhang P, Hu C, Ran W, Meng J, Yin Q, Li Y (2016b) Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics 6:948–968
Zheng DW et al (2017) Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Lett 17:284–291
Zhou Z et al (2017) Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem Int Ed Engl 56:6492–6496
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhang, X., Guo, S., Yang, Y., Xue, X., Wang, J. (2019). Ferroptosis in Cancer Therapy. In: Tang, D. (eds) Ferroptosis in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-26780-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-26780-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-26779-7
Online ISBN: 978-3-030-26780-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)