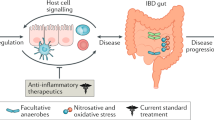
Abstract
Understanding of the pathogenesis of inflammatory bowel disease (IBD) continues to evolve. Current evidence suggests that IBD results from an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host. Dysbiosis, that is, unfavorable alteration of the composition and function of gastrointestinal (GI) microbiota or chronic pathogen infection, may be the mechanisms microbiota contribute to the pathogenesis of IBD. Several microbes have been associated with IBD, and among them, bacteria such as Clostridium species, gram-negatives, fecal microbes, and Mycobacterium avium subspecies remain exciting candidate pathogens but yet undiscovered other bacteria, viruses, and fungi also likely contribute significantly. Current therapeutic strategies targeting microbes that may be beneficial include probiotics and possibly fecal microbiota transplantation while dietary changes, prebiotics, and antibiotics have proven to be unhelpful. Newly discovered techniques focusing on molecular analysis of gut bacteria flora in combination with high genomic approaches are likely to further the insights into the role that microbes play in the development of IBD.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46.
Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15(6):857.
Porter CK, Tribble DR, Aliaga PA, Halvorson HA, Riddle MS. Infectious gastroenteritis and risk of develo** inflammatory bowel disease. Gastroenterology. 2008;135(3):781.
Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495.
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. https://doi.org/10.1056/NEJMra0804647.
Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66(4):515–22.
Kim DH, Cheon JH. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017;17(1):25–40.
Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8.
Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (NY). 2010;6(5):339–46.
Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50(5):495–507.
Scholz D. The role of nutrition in the etiology of inflammatory bowel disease. Curr Probl Pediatr Adolesc Health Care. 2011;41(9):248–53.
Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–89.
Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63–73.
Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62(10):1505–10.
Lidar M, Langevitz P, Shoenfeld Y. The role of infection in inflammatory bowel disease: initiation, exacerbation and protection. Isr Med Assoc J. 2009;11:558–63.
Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–21.
Burnham WR, Lennard-Jones JE, Stanford JL, Bird RG. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet. 1978;2:693–6.
Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut. 2008;57:850–60.
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–64.
Clayton EM, Rea MC, Shanahan F, Quigley EM, Kiely B, Hill C, Ross RP. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104:1162–9.
Monaghan TM, Cockayne A, Mahida YR. Pathogenesis of Clostridium difficile infection and its potential role in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1957–66.
Mahida YR, Makh S, Hyde S, et al. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–47.
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–90.
Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol. 2008;14:165–73.
Lundgren A, Strömberg E, Sjöling A, Lindholm C, et al. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori- infected patients. Infect Immun. 2005;73:523–31.
Rad R, Brenner L, Bauer S, Schwendy S, Layland L, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–37.
Watson GW, Fuller TJ, Elms J, Kluge RM. Listeria cerebritis: relapse of infection in renal transplant patients. Arch Intern Med. 1978;138:83–7.
Munoz P, Rojas L, Bunsow E, Saez E, et al. Listeriosis: an emerging public health problem especially among the elderly. J Infect. 2012;64:19–33.
Miranda-Bautista J, Padilla-Suarez C, Bouza E, Munoz P, Menchen L, Marın-Jimenez I. Listeria monocytogenes infection in inflammatory bowel dis- ease patients: case series and review of the literature. Eur J Gastroenterol Hepatol. 2014;26:1247–52.
Dalziel TK. Chronic interstitial enteritis. Br Med J. 1913;2:1068–70.
Olsen I, Tollefsen S, Aagaard C, et al. Isolation of Mycobacterium avium subspecies paratuberculosis reactive CD4 T cells from intestinal biopsies of Crohn’s disease patients. PLoS One. 2009;4:e5641.
Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–91.
Mpofu CM, Campbell BJ, Subramanian S, et al. Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn’s disease. Gastroenterology. 2007;133:1487–98.
Barreau F, Meinzer U, Chareyre F, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One. 2007;2:e523.
Elguezabal N, Chamorro S, Molina E, Garrido JM, Izeta A, Rodrigo L, et al. Lactase persistence, NOD2 status and Mycobacterium avium subsp. paratuberculosis infection associations to inflammatory bowel disease. Gut Pathog. 2012;4:6.
Kojima Y, Kinouchi Y, Takahashi S, Negoro K, Hiwatashi N, Shimosegawa T. Inflammatory bowel disease is associated with a novel promoter polymorphism of natural resistance-associated macrophage protein 1 (NRAMP1) gene. Tissue Antigens. 2001;58:379–84.
Juste RA, Elguezabal N, Pavón A, et al. Association between Mycobacterium avium subsp. paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Int J Infect Dis. 2009;13:247–54.
Macfarlane S, Furrie E, Cummings JH, Macfarlane GT. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin Infect Dis. 2004;38:1690–9.
Tabaqchali S, O’Donoghue DP, Bettelheim KA. Escherichia coli antibodies in patients with inflammatory bowel disease. Gut. 1978;19:108–13.
Johnson JR, Delavari P, Kuskowski M, Stell AL. Phylogenetic distribution of extraintestinal virulence- associated traits in Escherichia coli. J Infect Dis. 2001;183:78–88.
Petersen AM, Halkjær SI, Gluud LL. Intestinal colonization with phylogenetic group B2 Escherichia coli related to inflammatory bowel disease: a systematic review and meta-analysis. Scand J Gastroenterol. 2015;50:1199–207.
Navidinia M, Peerayeh SN, Fallah F, Bakhshi B, Sajadinia RS. Phylogenetic grou** and pathotypic comparison of urine and fecal Escherichia coli isolates from children with urinary tract infection. Braz J Microbiol. 2014;45:509–14.
Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127:80–93.
Barnich N, Carvalho FA, Glasser AL, Darcha C, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Investig. 2007;117:1566–74.
Conte MP, Longhi C, Marazzato M, Conte AL, et al. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn’s disease patients. BMC Res Notes. 2014;7:748.
Dogan B, Suzuki H, Herlekar D, Sartor RB, Campbell BJ, Roberts CL, et al. Inflammation-associated adherent-invasive Escherichia coli are enriched in pathways for use of propanediol and iron and M-cell translocation. Inflamm Bowel Dis. 2014;20:1919–32.
Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, Riordan SM, Grimm M, Leach ST, Ismail Y. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol. 2010;48:2965–7.
Zhang L, Man SM, Day AS, Leach ST, et al. Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn’s disease. J Clin Microbiol. 2009;47:453–5.
Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, Mitchell H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm Bowel Dis. 2010;16:1008–16.
Mahendran V, Riordan SM, Grimm MC, Tran TA, Major J, Kaakoush NO, Mitchell H, Zhang L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS One. 2011;6:e25417.
Zhang L, Lee H, Grimm MC, Riordan SM, Day AS, Lemberg DA. Campylobacter concisus and inflammatory bowel disease. World J Gastroenterol. 2014;20:1259–67.
Mahendran V, Tan YS, Riordan SM, Grimm MC, et al. The prevalence and polymorphisms of zonula occluden toxin gene in multiple Campylobacter concisus strains isolated from saliva of patients with inflammatory bowel disease and controls. PLoS One. 2013;8:e75525.
Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603.
Muller S, Arni S, Varga L, Balsiger B, Hersberger M, Maly F, et al. Serological and DNA-based evaluation of Chlamydia pneumoniae infection in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2006;18:889–94.
Chen W, Li D, Paulus B, Wilson I, Chadwick VS. High prevalence of Mycoplasma pneumoniae in intestinal mucosal biopsies from patients with inflammatory bowel disease and controls. Dig Dis Sci. 2001;46:2529–35.
Shibata KI, Hasebe A, Sasaki T, Watanabe T. Mycoplasma salivarium induces interleukin-6 and inter- leukin-8 in human gingival fibroblasts. FEMS Immunol Med Microbiol. 1997;19:275–83.
Baseman JB, Tully JG. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21.
Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated micro- biota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15:653–60.
Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–9.
Suau A, Bonnet R, Sutren M, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–807.
Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6.
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74.
Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31.
Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–44.
Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, Chieppa M, Arseneau KO, Ley K, Cominelli F. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–84.
Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45.
Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71.
Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62.
Merger M, Viney JL, Borojevic R, Steele-Norwood D, et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51:155–63.
Zhou P, Streutker C, Borojevic R, Wang Y, Croitoru K. IL-10 modulates intestinal damage and epithelial cell apoptosis in T cell-mediated enteropathy. Am J Physiol Gastrointest Liver Physiol. 2004;287:G599–604.
Little LM, Shadduck JA. Pathogenesis of rotavirus infection in mice. Infect Immun. 1982;38:755–63.
Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–9.
Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–31.
Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–23.
Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract. 2009;24:10–4.
Gionchetti P, Rizzello F, Venturi A, Brigidi P, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo- controlled trial. Gastroenterology. 2000;119:305–9.
Mimura T, Rizzello F, Helwig U, Poggioli G, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14.
Tursi A, Brandimarte G, Papa A, Giglio A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo- controlled study. Am J Gastroenterol. 2010;105:2218–27.
Sood A, Midha V, Makharia GK, Ahuja V, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9.
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–43.
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–9.
Kruis W, Fric P, Pokrotnieks J, Lukás M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–23.
Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–8.
Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–74.
Bousvaros A, Guandalini S, Baldassano RN, Botelho C, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–9.
Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–9.
Kato K, Mizuno S, Umesaki Y, Ishii Y, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–41.
Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63.
Laake KO, Bjørneklett A, Aamodt G, Aabakken L, Jacobsen M, Bakka A, Vatn MH. Outcome of four weeks’ intervention with probiotics on symptoms and endoscopic appearance after surgical reconstruction with a J-configurated ileal-pouch-anal-anastomosis in ulcerative colitis. Scand J Gastroenterol. 2005;40(1):43–51.
Bernstein CN. Antibiotics, probiotics and prebiotics in IBD. Nestle Nutr Inst Workshop Ser. 2014;79:83–100.
van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15.
Nitzan O, Elias M, Peretz A, Saliba W. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol. 2016;22(3):1078–87.
Ledder O, Turner D. Antibiotics in IBD: still a role in the biological era? Inflamm Bowel Dis. 2018;24(8):1676–88.
Balram B, Battat R, Al-Khoury A, D’Aoust J, Afif W, Bitton A, Lakatos PL, Bessissow T. Risk factors associated with Clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2019;13(1):27–38.
Llopis M, Antolin M, Carol M, Borruel N, et al. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15:275–83.
Wu X, Vallance BA, Boyer L, Bergstrom KS, et al. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294:G295–306.
Yan F, Cao H, Cover TL, Washington MK, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through EGFR-dependent mechanism. J Clin Invest. 2011;121:2242.
Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T. Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders. Curr Pharm Des. 2014;20(7):1149–55.
Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164.
Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota trans- plantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–16.
Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–30.
Kump PK, Gröchenig HP, Lackner S, Trajanoski S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–65.
Kunde S, Pham A, Bonczyk S, Crumb T, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601.
Qazi T, Amaratunga T, Barnes EL, Fischer M, Kassam Z, Allegretti JR. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: systematic review and meta-analysis. Gut Microbes. 2017;8(6):574–88.
Khan KJ, Ullman TA, Ford AC, Abreu MT, et al. Antibiotic therapy in inflammatory bowel disease: a systemic review and meta-analysis. Am J Gastroenterol. 2011;106:661–73.
Aleksandar D, Xavier RJ, Gevers D. The microbiome in inflammatory bowel diseases: current status and the future ahead. Gastroenterology. 2014;146(6):1489–99.
Zimmer J, Hofsø D, Aasheim ET, Tanbo T, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66:53–6.
LeBlanc JG, Laiño JE, del Valle MJ, Vannini V, et al. B-group vitamin production by lactic acid bacteria--current knowledge and potential applications. J Appl Microbiol. 2011;111(6):1297–309.
Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. https://doi.org/10.1007/s00281-014-0454-4. Epub 2014 Nov 25.
Bouskra D, Brézillon C, Bérard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–10.
Conflicts of Interest
The authors have no conflicts of interest.
There was no funding for this book chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jatwani, S., Malhotra, B., Crout, T., Majithia, V. (2019). Microbes in the Pathogenesis of Inflammatory Bowel Disease: A Review. In: Espinoza, L. (eds) Infections and the Rheumatic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-23311-2_37
Download citation
DOI: https://doi.org/10.1007/978-3-030-23311-2_37
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23310-5
Online ISBN: 978-3-030-23311-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)