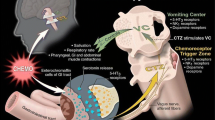
Abstract
Oncology practitioners currently have very effective antiemetic agents in the form of 5-hydroxytryptamine-3 receptor antagonists, neurokinin-1 receptor antagonists, dexamethasone, and olanzapine for use in the prevention of chemotherapy-induced nausea and vomiting in patients receiving moderately or highly emetogenic chemotherapy. The choice of individual agents and the combination of agents should be dictated by the emetogenicity of the chemotherapy and patient risk factors. The available agents for the prevention of CINV appear to be safe and effective with few reported adverse events when used in the recommended doses.
The use of these agents in various clinical settings is described by established antiemetic guidelines from the Multinational Association of Supportive Care in Cancer and the European Society of Medical Oncology, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network. These guidelines should be followed by practitioners in order to provide the highest possible quality of care for patients receiving chemotherapy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ASCO:
-
American Society of Clinical Oncology
- CINV:
-
Chemotherapy-induced nausea and vomiting
- CTZ:
-
Chemoreceptor trigger zone
- FDA:
-
Food & Drug Administration
- GI:
-
Gastrointestinal
- 5-HT3:
-
5-hydroxytryptamine-3
- HEC:
-
Highly emetogenic chemotherapy
- MEC:
-
Moderately emetogenic chemotherapy
- MASCC:
-
Multinational Association of Supportive Care in Cancer
- NCCN:
-
National Comprehensive Cancer Network
- NTS:
-
Nucleus Tractus solitarius
- NK-1:
-
Neurokinin-1
- VAS:
-
Visual analogue scale
- VC:
-
Vomiting Centre
References
Bloechl-Daum B et al (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Cohen L et al (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Navari RM, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374:1356–1367
Grunberg SM (2004) Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment—how are we doing? J Support Oncol 2:1–10
Broder MS et al (2014) The impact of 5HT3RA use on cost and utilization in patients with chemotherapy-induced nausea and vomiting: systematic review of the literature. Am Health Drug Benefits 7:171–182
Navari RM (2013) Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 73:249–262
Navari RM (2014) Palonosetron for the treatment of chemotherapy-induced nausea and vomiting. Expert Opin Pharmacother 15:2599–2608
Aapro M, Carides A, Rapoport BL (2015) Aprepitant and fosaprepitant: a ten-year review of efficacy and safety. Oncologist 20:450–458
Navari RM (2015) Profile of netupitant/palonosetron fixed dose combination (NEPA) and its potential in the treatment of chemotherapy-induced nausea and vomiting (CINV). Drug Des Devel Ther 9:155–161
Navari RM (2015) Rolapitant for the treatment of chemotherapy induced nausea and vomiting. Expert Rev Anticancer Ther 15:1127–1133
Navari RM et al (2007) A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting. Support Care Cancer 15:1285–1291
Tan L et al (2009) Clinical research of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 28:1–7
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195
Navari RM et al (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134–142
Mukhopadhyay S et al (2017) Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 25:145–154
Navari RM (2012) Treatment of chemotherapy-induced nausea. Commun Oncol 9:20–26
Ng TL, Hutton B, Clemons M (2015) Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncologist 20:576–583
Stern RM, Koch KL, Andrews PLR (2011) Nausea: mechanisms and management. Oxford University Press, New York
Molassiotis A et al (2017) MASCC/ESMO antiemetic guidelines: introduction to the 2016 guideline update. Support Care Cancer 25:267
Hesketh PJ et al (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240–3261
NCCN Clinical Practice Guidelines in Oncology version 2.2017. Antiemesis. National Comprehensive Cancer Network (NCCN) [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Accessed June, 2017
Koga T, Fukuda H (1992) Neurons in the nucleus of the solitary tract mediating inputs from Vagal afferents and the area postrema in the pattern generator in the emetic act in dogs. Neurosci Res 14:366–379
Yates BJ et al (1994) Organization of the vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Phys 267:974–983
Simpson K, Spencer CM, McClellan KJ (2000) Topisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 59:1297–1315
Kimura E et al (1996) Study on clinical effect of a continuous intravenous infusion of azasetron against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho 23:477–481
Taguchi T et al (1999) Usefulness of ramosetron hydrochloride on nausea and vomiting in CMF or CEF therapy for breast cancer. Gan To Kagaku Ryoho 26:1163–1170
Hesketh PJ (2000) Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Investig 18:163–173
World Health Organization (2006) Dolasetron mesylate and serious cardiovascular reactions, vol 20. WHO Drug Information, p 185
U.S. Food and Drug Information. FDA drug safety communication: abnormal heart rhythms associated with use of anzemet (dolasetron mesylate) [online]. Available from URL: http://www.fda.gov/Drugs.DrugSafety/usm237081.htm. Accessed 15 June 2017
Ondansetron [online], Available from URL: www.drugs.com/fda/ondansetron. Accessed 15 June July 2017
Mason JW et al (2014) A randomized, placebo-controlled, four-period cross-over definitive QT study of the effects of APF530 exposure, high-dose intravenous granisetron, and moxifloxacin on QTC prolongation. Cancer Manag Res 6:183–189
Roila F et al (2005) Delayed emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:104–108
Geling O, Eichler H (2005) Should 5-Hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Hickok JT et al (2005) 5-HT3 receptor antagonists versus perchlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomized controlled trial. Lancet Oncol 6:765–772
Saito M et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for the prevention of nausea and vomiting during chemotherapy: a double-blind, double dummy, randomized, comparative phase III trial. Lancet Oncol 10:115–124
Warr DG et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830
Boccia RV et al (2011) Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 19:1609–1617
Raftopoulos H et al (2015) Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double blind, noninferiority phase III trial. Support Care Cancer 23:723–732
Eisenberg P et al (2004) Efficacy, safety, and pharmacokinetics of palonosetron in patients receiving highly emetogenic, cisplatin-based chemotherapy: a dose-ranging, clinical study. Ann Oncol 15:330–337
Rojas C et al (2010) Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. J Pharmacol 626:193–199
Botrel T et al (2011) Efficacy of palonosetron compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic treatment: systematic review and meta-analysis. Support Care Cancer 19:823–832
Schwartzberg L et al (2014) Pooled analysis of phase III studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting. Support Care Cancer 22:469–477
Popovic M et al (2014) Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 22:1485–1497
Yavas C et al (2012) Acute effect of palonosetron electrocardiographic parameters in cancer patients; a prospective study. Support Care Cancer 20:2343–2347
Aogi K et al (2012) A phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapy. Support Care Cancer 20:1507–1514
Roila F et al (2014) Aprepitant versus metoclopramide, both combined with dexamethasone, for the preventing cisplatin-induced delayed emesis: a randomized, double-blind study. J Clin Oncol 32(5s (suppl)):abstr 9503
Navari RM, Nagy CK, Gray SE (2013) Olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21:1655–1663
European Medicine Agency Metoclopramide use recommendations, 26 July 2013. www.ema.europa.eu. Accessed 15 June 2017
Diemunsch P, Grelot L (2000) Potential of substance P antagonists as antiemetics. Drugs 60:533–546
Sankhala KK et al (2009) Prevention of chemotherapy induced nausea and vomiting: a focus on aprepitant. Expert Opin Drug Metab Toxicol 12:1607–1614
Hesketh PJ et al (2006) Combined data from two phase III trials of the NK-1 antagonist aprepitant plus a 5HT3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer 14:354–360
Warr DG et al (2005) The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from two randomized, double-blind, placebo controlled trials. Eur J Cancer 41:1278–1285
Grote T et al (2006) Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol 4:403–408
Navari RM (2007) Fosaprepitant (MK-0517): a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Investig Drugs 16:1977–1985
Grunberg S et al (2011) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol 29:1495–1501
Leal AD et al (2014) Fosaprepitant-induced phlebitis: a focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer 22:1313–1317
Drug interactions of aprepitant [slide presentation]. Available at http://www.fda.gov/ohrms/dockets/ac/03/slides/3928s1_03_fda-jarugula.ppt. Accessed 9 June 2017
Scientific discussion: this module reflects the initial scientific discussion for the approval of Emend. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Scientific_Discussion/human/000527/WC500026534.pdf. Accessed 9 June 2017
Aapro MS, Walko CM (2010) Aprepitant: drug-drug interactions in perspective. Ann Oncol 21:2316–2323
Schmitt T et al (2014) Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. J Clin Oncol 32:3413–3420
Pielichowski W et al (2011) A triple-drug combination to prevent nausea and vomiting following BEAM chemotherapy before autologous hematopoietic stem cell transplantation. Transplant Proc 43:3107–3110
Stiff PJ et al (2013) Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant 19:49–55
Svanberg A, Bergegard C (2015) Addition of aprepitant to standard antiemetic regimen continued for seven days after chemotherapy for stem cell transplantation provide significant reduction of vomiting. Oncologia 89:31–36
Uchida M et al (2013) Effectiveness and safety of antiemetic aprepitant in Japanese patients receiving high-dose chemotherapy prior to autologous hematopoietic stem cell transplantation. Biol Pharm Bull 36:819–824
Sakuri M et al (2014) Efficacy of aprepitant in preventing nausea and vomiting due to high-dose melphalan–based conditioning for allogeneic hematopoietic stem cell transplantation. Int J Hematol 99:457–462
Bechtel T et al (2014) Aprepitant for the control of delayed nausea and vomiting associated with the use of high-dose melphalan for autologous peripheral blood stem cell transplants in patients with multiple myeloma: a phase II study. Support Care Cancer 22:2911–2916
Einhorn LH et al (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer 25:303–308
Ottoboni T et al (2017) Bioequivalence of HTX-019 (aprepitant IV)(Cinvanti) and fosaprepitant in healthy subjects. Hematology/Oncology Pharmacy Association Annual Conference, March 29–April 1, 2017, Anaheim, CA
Rizzi A et al (2012) In vitro and in vivo pharmacological characterization of the novel NK-1 receptor selective antagonist Netupitant. Peptides 37:86–97
Spinelli T, Calcagneli S, Giuliano C (2014) Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol 54:97–108
National Cancer Institute Drug Dictionary. Available from http://www.cancer.gov/drugdictionary. Accessed 15 June 2017
Hesketh PJ et al (2014) Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 25:1340–1346
Aapro M et al (2014) A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25:1328–1333
Gralla RJ et al (2014) A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 25:1333–1339
FDA News Release. FDA approves Akynzeo for nausea and vomiting associated with cancer chemotherapy. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements. Accessed 9 June 2017
Duffy RA et al (2012) Rolapitant (SCH 619734): a potent, selective and orally active neurokinin NK-1 receptor antagonist with centrally-mediated antiemetic effects in ferrets. Pharmacol Biochem Behav 102:95–100
Poma A et al (2013) Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Support Care Cancer 21:S154. Abstract 441
Poma A et al (2014) Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol 32:abstr e20690
Rapoport B et al (2015) Study of rolapitant, a novel, long acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer 23:3281–3288
Schwartzberg L et al (2015) Safety and efficacy assessment of rolapitant for the prevention of chemotherapy-induced nausea and vomiting following administration of moderately emetogenic chemotherapy in cancer patients in a randomized phase 3 trial. Lancet Oncol 16:1071–1078
Rapoport B et al (2015) Safety and efficacy assessment of rolapitant for the prevention of chemotherapy-induced nausea and vomiting following administration of cisplatin-based highly emetogenic chemotherapy in cancer patients in two randomized phase 3 trials. Lancet Oncol 16:1079–1089
dos Santos LV et al (2012) Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl Cancer Inst 104:1280–1292
Zhang Y et al (2016) Neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting: a network meta-analysis. J Natl Cancer Inst 109:djw217. https://doi.org/10.1093/jnci/djw217
Vardy J et al Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 1999, 94:1011–1015
Celio L et al (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multi-center, phase III trial. Support Care Cancer 19:1217–1225
Aapro M et al (2010) Double-blind, randomized, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–1088
Fulton B, Goa KL (1999) Olanzapine: a review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs 53:281–298
Kando JC et al (1997) Olanzapine: a new antipsychotic agent with efficacy in the management of schizophrenia. Ann Pharmacother 31:1325–1334
Bymaster FP et al (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Stephenson CME, Pilowski LS (1999) Psychopharmacology of olanzapine: a review. Br J Psychiatry 174:52–58
Petersen AB et al (2014) Adverse effects associated with high dose therapy in patients admitted to inpatient psychiatric care. Clin Toxicol 52:39–43
Passik S et al (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients receiving chemotherapy. Cancer Investig 22:383–388
Goldstein LE et al (1999) New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 40:438–443
Mizukami N et al (2014) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind placebo-controlled study. J Pain Symptom Manag 47:542–550
Davis MP (2016) New therapies for antiemetic prophylaxis for chemotherapy. J Community Support Oncol 14:11–20
Irving G et al (2009) Efficacy and tolerability of gastric-retentive gabapentin for the treatment of post-herpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin J Pain 25:185–192
Guttuso T, Roscoe J, Griggs J (2003) Effect of gabapentin on nausea induced by chemotherapy in patients with breast cancer. Lancet 361:1703–1705
Cruz FM et al (2011) Gabapentin for the prevention of chemotherapy-induced nausea and vomiting: a pilot study. Support Care Cancer 20:601–606
Barton DL et al (2014) Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance). Cancer 120:3575–3583
Van Sickle MD et al (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Darmani NA (2001) Delta-9-tetrahydrocannabinol differentially supresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol Biochem Behav 69:239–249
Meiri E et al (2007) Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin 23:533–543
Davis MP (2008) Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin Investig Drugs 17:85–95
May MB, Grode AE (2016) Dronabinaol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res 8:49–55
Badowski ML (2017) A review of oral cannabinoids and medical marijuana for the treatment of CINV: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother Pharmacol 80:441–449
Pillai AK et al (2011) Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving highly emetogenic chemotherapy. Pediatr Blood Cancer 56:234–238
Zick SM et al (2009) Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 17:563–572
Ryan JL et al (2012) Ginger reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer 20:1479–1489
Bossi P et al (2016) Searching for evidence to support the use of ginger in the prevention of chemotherapy-induced nausea and vomiting. J Altern Complement Med 22:486–488
Geling O, Eichler H (2005) Should 5-Hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Navari RM (2016) Should behavioral therapy be used as the primary treatment to control anticipatory vomiting? HemeOnc Today 17:13
Navari RM (2017) Cancer patients at high risk for chemotherapy-induced nausea and vomiting – prediction assessments/tools. Expert Rev Qual Cancer Care 2:102–108
Zhang L et al (2017) A randomized phase 3 study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly Emetogenic chemotherapy (HEC). Ann Oncol 29:452–458. https://doi.org/10.1093/annonc/mdx698
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Navari, R.M. (2019). Chemotherapy Induced Nausea and Vomiting. In: De Mello, R., Mountzios, G., Tavares, Á. (eds) International Manual of Oncology Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-16245-0_46
Download citation
DOI: https://doi.org/10.1007/978-3-030-16245-0_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16244-3
Online ISBN: 978-3-030-16245-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)