Abstract
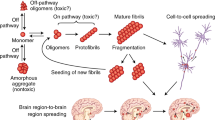
Prions and other self-propagating protein aggregates can exist as distinct strains, which are thought to represent different conformations of aggregates. There is growing evidence that protein aggregate strains may be important for understanding the biology of common neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease. While methodology for discriminating prion strains is in widespread use, there is a paucity of tools for comparing the conformational properties of aggregates composed of β-amyloid (Aβ) peptide or α-synuclein protein, particularly when present in complex samples such as brain extracts. The conformational stability assay (CSA) is a simple technique that measures the relative resistance of protein aggregates to chemical denaturation. While originally developed to differentiate prion strains, the CSA has since been adapted for use with other protein aggregates. Here, we describe the CSA in detail and outline its utility for distinguishing prion strains as well as unique conformational states of Aβ and α-synuclein aggregates.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Prusiner SB (2012) A unifying role for prions in neurodegenerative diseases. Science 336:1511–1513
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51
Guo JL, Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20(2):130–138
Goedert M (2015) NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 349(6248):1255555
Colby DW, Prusiner SB (2011) Prions. Cold Spring Harb Perspect Biol 3:a006833
Polymenidou M, Cleveland DW (2012) Prion-like spread of protein aggregates in neurodegeneration. J Exp Med 209:889–893
Aguzzi A, Rajendran L (2009) The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64:783–790
Hardy J, Revesz T (2012) The spread of neurodegenerative disease. N Engl J Med 366:2126–2128
Walker LC, Jucker M (2015) Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci 38:87–103
Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318:930–936
Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R (2005) Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 307:262–265
Paravastu AK, Qahwash I, Leapman RD, Meredith SC, Tycko R (2009) Seeded growth of beta-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc Natl Acad Sci U S A 106:7443–7448
Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R (2013) Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154(6):1257–1268
Heilbronner G, Eisele YS, Langer F, Kaeser SA, Novotny R, Nagarathinam A, Aslund A, Hammarstrom P, Nilsson KP, Jucker M (2013) Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. EMBO Rep 14(11):1017–1022
Watts JC, Condello C, Stohr J, Oehler A, Lee J, DeArmond SJ, Lannfelt L, Ingelsson M, Giles K, Prusiner SB (2014) Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci U S A 111(28):10323–10328
Stohr J, Condello C, Watts JC, Bloch L, Oehler A, Nick M, DeArmond SJ, Giles K, DeGrado WF, Prusiner SB (2014) Distinct synthetic Abeta prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci U S A 111(28):10329–10334
Cohen ML, Kim C, Haldiman T, ElHag M, Mehndiratta P, Pichet T, Lissemore F, Shea M, Cohen Y, Chen W, Blevins J, Appleby BS, Surewicz K, Surewicz WK, Sajatovic M, Tatsuoka C, Zhang S, Mayo P, Butkiewicz M, Haines JL, Lerner AJ, Safar JG (2015) Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-beta. Brain 138(Pt 4):1009–1022
Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM (2013) Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154:103–117
Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Bockmann A, Meier BH, Melki R (2013) Structural and functional characterization of two alpha-synuclein strains. Nat Commun 4:2575
Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A 110(48):19555–19560
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V (2015) alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522(7556):340–344
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K (2015) Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A 112(38):E5308–E5317
Peretz D, Scott M, Groth D, Williamson A, Burton D, Cohen FE, Prusiner SB (2001) Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10:854–863
Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton D, DeArmond SJ, Prusiner SB, Scott MR (2002) A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921–932
Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB (2006) Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci U S A 103:19105–19110
Watts JC, Giles K, Patel S, Oehler A, Dearmond SJ, Prusiner SB (2014) Evidence That bank vole PrP is a universal acceptor for prions. PLoS Pathog 10(4):e1003990
Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, Jewell J, Graham C, Hoover EA, Telling GC (2010) Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328:1154–1158
Safar JG, Scott M, Monaghan J, Deering C, Didorenko S, Vergara J, Ball H, Legname G, Leclerc E, Solforosi L, Serban H, Groth D, Burton DR, Prusiner SB, Williamson RA (2002) Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat Biotechnol 20:1147–1150
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164
Lee BR, Kamitani T (2011) Improved immunodetection of endogenous α-synuclein. PLoS One 6:e23939
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Lau, H.H.C., Lau, A., Watts, J.C. (2018). Discriminating Strains of Self-Propagating Protein Aggregates Using a Conformational Stability Assay. In: Nilsson, B., Doran, T. (eds) Peptide Self-Assembly. Methods in Molecular Biology, vol 1777. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7811-3_22
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7811-3_22
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7809-0
Online ISBN: 978-1-4939-7811-3
eBook Packages: Springer Protocols