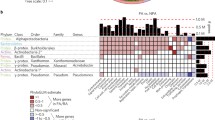
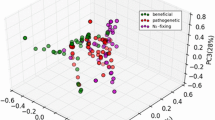
Abstract
In this introductory chapter, we briefly characterize major groups of plant-associated bacteria and discuss what is currently known about genome organization and plasticity in such versatile and resourceful microbes as plant pathogenic and plant-growth-promoting bacteria.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Allardet-Servent A, Michaux-Charachon S, Jumas-Bilak E et al (1993) Presence of one linear and one circular chromosome in the Agrobacterium tumefaciens C58 genome. J Bacteriol 175:7869–7874
Amadou C, Pascal G, Mangenot S et al (2008) Genome sequence of the β-rhizobium Cupriavidus taiwanensis and comparative genomics of rhizobia. Genome Res 18:1472–1483
Amato P, Parazols M, Sancelme M et al (2007) Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dôme: major groups and growth abilities at low temperatures. FEMS Microbiol Ecol 59:242–254
Antoun H, Prévost D (2005) Ecology of plant growth promoting rhizobacteria. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 1–38
Antoun H, Beauchamp CJ, Goussard N et al (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
Arnold DL, Jackson RW, Waterfield NR, Mansfield JW (2007) Evolution of microbial virulence: the benefits of stress. Trends Genet 23:293–300
Bakkali M (2013) Could DNA uptake be a side effect of bacterial adhesion and twitching motility? Arch Microbiol 195:279–289
Baldani JI, Caruso L, Baldani VLD et al (1997) Recent advances in BNF with non-legume plants. Soil Biol Biochem 29:911–922
Baltrus DA, Nishimura MT, Romanchuck A et al (2011) Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19 Pseudomonas syringae isolates. PLoS Pathog 7:e1002132
Baril C, Richaud C, Baranton G, Saint-Girons I (1989) Linear chromosome of Borrelia burgdorferi. Res Microbiol 140:507–516
Batut J, Andersson SG, O’Callaghan D (2004) The evolution of chronic infection strategies in the alpha-proteobacteria. Nat Rev Microbiol 2:933–945
Bender CL, Cooksey DA (1986) Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol 165:534–541
Benson DR, Brooks JM, Huang Y et al (2011) The biology of Frankia sp. strains in the post-genome era. Mol Plant Microbe Interact 24:1310–1316
Bentley SD, Parkhill J (2004) Comparative genomic structure of prokaryotes. Annu Rev Genet 38:771–792
Berg G, Eberl L, Hartmann A (2005) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685
Bertalan M, Albano R, de Pádua V et al (2009) Complete genome sequence of the sugarcane nitrogen-fixing endophyte Gluconacetobacter diazotrophicus Pal5. BMC Genomics 10:450
Björklöf K, Nurmiaho-Lassila EL, Klinger N et al (2000) Colonization strategies and conjugal gene transfer of inoculated Pseudomonas syringae on the leaf surface. J Appl Microbiol 89:423–432
Bladergroen MR, Badelt K, Spaink HP (2003) Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol Plant Microbe Interact 16:53–64
Boucher C, Martinel A, Barberis P et al (1986) Virulence genes are carried by a megaplasmid of the plant pathogen Pseudomonas solanacearum. Mol Gen Genet 205:270–275
Boussau B, Karlberg EO, Frank AC et al (2004) Computational inference of scenarios for alpha-proteobacterial genome evolution. Proc Natl Acad Sci U S A 101:9722–9727
Bull CT, De Boer SH, Denny TP et al (2010) Comprehensive list of names of plant pathogenic bacteria, 1980–2007. J Plant Pathol 92:551–592
Bull CT, De Boer SH, Denny TP et al (2012) List of new names of plant pathogenic bacteria (2008–2010). J Plant Pathol 94:21–27
Burrus V, Pavlovic G, Decaris B, Guédon G (2002) Conjugative transposons: the tip of the iceberg. Mol Microbiol 46:601–610
Cascales E (2008) The type VI secretion toolkit. EMBO Rep 98:735–741
Chaintreuil C, Giraud E, Prin Y et al (2000) Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol 66:5437–5447
Chan KG, Atkinson S, Mathee K et al (2011) Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol 11:51
Chen CW (1996) Complications and implications of linear bacterial chromosomes. Trends Genet 12:192–196
Chibani-Chennoufi S, Bruttin A, Dillmann ML, Brüssow H (2004) Phage-host interaction: an ecological perspective. J Bacteriol 186:3677–3686
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Claverys JP, Martin B, Polard P (2009) The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol Rev 33:643–656
Crespi M, Messens E, Caplan AB et al (1992) Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J 11:795–804
Deakin WJ, Broughton WJ (2009) Symbiotic use of pathogenic strategies: rhizobial protein secretion systems. Nat Rev Microbiol 7:312–320
del Carmen Orozco-Mosqueda M, Altamirano-Hernandez J, Farias-Rodriguez R et al (2009) Homologous recombination and dynamics of rhizobial genomes. Res Microbiol 160:733–741
Demanèche S, Kay E, Gourbière F, Simonet P (2001) Natural transformation of Pseudomonas fluorescens and Agrobacterium tumefaciens in soil. Appl Environ Microbiol 67:2617–2621
Ding H, Hynes MF (2009) Plasmid transfer systems in the rhizobia. Can J Microbiol 55:917–927
Dinoor A, Eshed N (1984) The role and importance of pathogens in natural plant communities. Annu Rev Phytopathol 22:443–466
Dobrindt U, Hochhut B, Hentschel U, Hacker J (2004) Genomic islands in pathogenic and environmental microorganisms. Nat Rev Microbiol 2:414–424
El Yacoubi B, Brunings AM, Yuan Q et al (2007) In planta horizontal transfer of a major pathogenicity effector gene. Appl Environ Microbiol 73:1612–1621
Espinosa-Urgel M (2004) Plant-associated Pseudomonas populations: molecular biology, DNA dynamics, and gene transfer. Plasmid 52:139–150
Faure D, Vereecke D, Leveau JHJ (2009) Molecular communication in the rhizosphere. Plant Soil 321:279–303
Ferdows MS, Barbour AG (1989) Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A 86:5969–5973
Ferrante P, Scortichini M (2013) Frost promotes the pathogenicity of Pseudomonas syringae pv. actinidiae in Actinidia chinensis and A. deliciosa plants. Plant Pathol. doi:10.1111/ppa.12070
Filonov AE, Akhmetov LI, Puntus IF et al (2005) The construction and monitoring of genetically tagged, plasmid-containing, naphthalene-degrading strains in soil. Microbiology 74:453–458
Fletcher J, Leach JE, Eversole K, Tauxe R (2013) Human pathogens on plants: designing a multidisciplinary strategy for research. Phytopathology 103:306–315
Fouts DE, Tyler HL, DeBoy RT et al (2008) Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genet 4:e1000141
Francis I, Holsters M, Vereecke D (2010) The Gram-positive side of plant–microbe interactions. Environ Microbiol 12:1–12
Frost LS, Leplae R, Summers AO, Toussaint A (2005) Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 3:722–732
Galardini M, Mengoni A, Brilli M et al (2011) Exploring the symbiotic pangenome of the nitrogen-fixing bacterium Sinorhizobium meliloti. BMC Genomics 12:235
Gandon S, Van Baalen M, Jansen VA (2002) The evolution of parasite virulence, superinfection, and host resistance. Am Nat 159:658–669
Garavaglia BS, Thomas L, Gottig N et al (2010) A eukaryotic-acquired gene by a biotrophic phytopathogen allows prolonged survival on the host by counteracting the shut-down of plant photosynthesis. PLoS One 5:e8950
Garcillán-Barcia MP, Alvarado A, de la Cruz F (2011) Identification of bacterial plasmids based on mobility and plasmid population biology. FEMS Microbiol Rev 35:936–956
Gazi AD, Sarris PF, Fadouloglou VE et al (2012) Phylogenetic analysis of a gene cluster encoding an additional, rhizobial-like type III secretion system that is narrowly distributed among Pseudomonas syringae strains. BMC Microbiol 12:188
Ghinet MG, Bordeleau E, Beaudin J et al (2011) Uncovering the prevalence and diversity of integrating conjugative elements in actinobacteria. PLoS One 6:e27846
Giongo A, Tyler HL, Zipperer UN, Triplett EW (2010) Two genome sequences of the same bacterial strain, Gluconacetobacter diazotrophicus PAl5, suggest a new standard in genome sequence submission. Stand Genomic Sci 2:309–317
González V, Santamaria RI, Bustos P et al (2006) The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc Natl Acad Sci U S A 103:3834–3839
Goodner B, Hinkle G, Gattung S et al (2001) Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323–2328
Gophna U, Ron EZ, Graur D (2003) Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events. Gene 312:151–163
Gyaneshwar P, Hirsch AM, Moulin L et al (2011) Legume nodulating beta-proteobacteria: diversity, host range, and future prospects. Mol Plant Microbe Interact 24:1276–1288
Hauberg-Lotte L, Klingenberg H, Scharf C et al (2012) Environmental factors affecting the expression of pilAB as well as the proteome and transcriptome of the grass endophyte Azoarcus sp. strain BH72. PLoS One 7:e30421
Heuer H, Smalla K (2007) Horizontal gene transfer between bacteria. Environ Biosafety Res 6:3–13
Hirano SS, Upper CD (2000) Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev 64:624–653
Hirsch AM, Valdés M (2009) Micromonospora: an important microbe for biomedicine and potentially for biocontrol and biofuels. Soil Biol Biochem 42:536–542
Hurek T, Reinhold-Hurek B (2003) Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol 106:169–178
Hurek T, Handley L, Reinhold-Hurek B, Piché Y (2002) Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol Plant Microbe Interact 15:233–242
Kamnev AA (2008) Metals in soil versus plant-microbe interactions: biotic and chemical interferences. In: Barka EA, Clément C (eds) Plant–microbe interactions. Research Signpost, Kerala, India, pp 291–318
Kaneko T, Nakamura Y, Sato S et al (2002) Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res 9:189–197
Kaneko T, Minamisawa K, Isawa T et al (2010) Complete genomic structure of the cultivated rice endophyte Azospirillum sp. B510. DNA Res 17:37–50
Katsy EI (2011) Plasmid plasticity in the plant-associated bacteria of the genus Azospirillum. In: Maheshwari DK (ed) Bacteria in agrobiology: plant growth responses. Springer, Berlin, pp 139–157
Kim JF, Charkowsky AO, Alfano JR (1998) Sequences related to transposable elements and bacteriophages flank avirulence genes of Pseudomonas syringae. Mol Plant Microbe Interact 11:1247–1252
Kim YC, Leveau J, McSpadden Gardener BB et al (2011) The multifactorial basis for plant health promotion by plant-associated bacteria. Appl Environ Microbiol 77:1548–1555
Kraiser T, Gras DE, Gutiérrez AG et al (2011) A holistic view of nitrogen acquisition in plants. J Exp Bot 62:1455–1466
Krause A, Ramakumar A, Bartels D et al (2006) Complete genome of the mutualistic, N2-fixing grass endophyte Azoarcus sp. strain BH72. Nat Biotechnol 24:1385–1391
Kube M, Schneider B, Kuhl H et al (2008) The linear chromosome of the plant-pathogenic mycoplasma ‘Candidatus Phytoplasma mali’. BMC Genomics 9:306
Kung SH, Almeida RP (2011) Natural competence and recombination in the plant pathogen Xylella fastidiosa. Appl Environ Microbiol 77:5278–5284
Landeta C, Dávalos A, Cevallos MA et al (2011) Plasmids with a chromosome-like role in rhizobia. J Bacteriol 193:1317–1326
Lang AS, Zhaxybayeva O, Beatty JT (2013) Gene transfer agents: phage-like elements of genetic exchange. Nat Rev Microbiol 10:472–482
Lau JA, Bowling EJ, Gentry LE et al (2012) Direct and interactive effects of light and nutrients on the legume-rhizobia mutualism. Acta Oecol 39:80–86
Lee A, Hirsch AM (2006) Signals and responses: choreographing the complex interaction between legumes and α- and β-Rhizobia. Plant Signal Behav 1:161–168
Levy-Booth DJ, Campbell RG, Gulden RH et al (2007) Cycling of extracellular DNA in the soil environment. Soil Biol Biochem 39:2977–2991
Lilley AK, Bailey MJ (1997) The acquisition of indigenous plasmids by a genetically marked pseudomonad population colonizing the sugar beet phytosphere is related to local environment conditions. Appl Environ Microbiol 63:1577–1583
Llop P, Cabrefiga J, Smits TH et al (2011) Erwinia amylovora novel plasmid pE170: complete sequence, biogeography, and role in aggressiveness in the fire blight phytopathogen. PLoS One 6:e28651
Llop P, Barbé S, López MM (2012) Functions and origin of plasmids in Erwinia species that are pathogenic to or epiphytically associated with pome fruit trees. Trees 26:31–46
Loper JE, Hassan KA, Mavrodi DV et al (2012) Comparative genomics of plant-associated Pseudomonas spp: insights into diversity and inheritance traits involved in multitrophic interactions. PLoS Genet 8:e1002784
Lotareva OV, Prozorov AA (2005) The conjugal transfer of plasmid pUB110 in Bacillus subtilis in soils of different natural landscapes. Microbiology 74:116–117
Lovell HC, Mansfield JW, Godfrey SAC et al (2009) Bacterial evolution by genomic island transfer occurs via DNA transformation in planta. Curr Biol 19:1586–1590
Madsen JS, Burmølle M, Hansen LH, Sørensen SJ (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65:183–195
Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104:1539–1551
Maheshwari DK (ed) (2011) Bacteria in agrobiology: plant growth responses. Springer, Berlin
Mahillon J, Chandler M (1998) Insertion sequences. Microbiol Mol Biol Rev 62:725–774
Manulis S, Barash I (2003) Pantoea agglomerans pvs. gypsophilae and betae, recently evolved pathogens? Mol Plant Pathol 4:307–314
Marchetti M, Capela D, Glew M et al (2010) Experimental evolution of a plant pathogen into a legume symbiont. PLoS Biol 8:e1000280
Marchi M, Boutin M, Gazengel K et al (2013) Genomic analysis of the biocontrol strain Pseudomonas fluorescens Pf29Arp with evidence of T3SS and T6SS gene expression on plant roots. Environ Microbiol Rep 5:393–403
Marie C, Broughton WJ, Deakin WJ (2001) Rhizobium type III secretion systems: legume charmers or alarmers? Curr Opin Plant Biol 4:336–342
Marques MV, da Silva AM, Gomes SL (2001) Genetic organization of plasmid pXF51 from the plant pathogen Xylella fastidiosa. Plasmid 45:184–199
Marri PR, Harris LK, Houmiel K (2008) The effect of chromosome geometry on genetic diversity. Genetics 179:511–516
Martin-Didonet CCG, Chubatsu LS, Souza EM et al (2000) Genome structure of the genus Azospirillum. J Bacteriol 182:4113–4116
Mela F, Fritsche K, Boersma H et al (2008) Comparative genomics of the pIPO2/pSB102 family of environmental plasmids: sequence, evolution, and ecology of pTer331 isolated from Collimonas fungivorans Ter331. FEMS Microbiol Ecol 66:45–62
Mew TW (1987) Current status and future prospects of research on bacterial blight of rice. Annu Rev Phytopathol 25:359–382
Mhedbi-Hajri N, Hajri A, Boureau T et al (2013) Evolutionary history of the plant pathogenic bacterium Xanthomonas axonopodis. PLoS One 8:e58474
Mølbak L, Licht TR, Kvist T et al (2003) Plasmid transfer from Pseudomonas putida to the indigenous bacteria on alfalfa sprouts: characterization, direct quantification, and in situ location of transconjugant cells. Appl Environ Microbiol 69:5536–5542
Monteiro-Vitorello CB, de Oliveira MC, Zerillo MM et al (2005) Xylella and Xanthomonas mobil’omics. OMICS 9:146–159
Morris CE, Sands DC, Vinatzer BA et al (2008) The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J 2:321–334
Nadarasah G, Stavrinides J (2011) Insects as alternative hosts for phytopathogenic bacteria. FEMS Microbiol Rev 35:555–575
NCBI Taxonomy. http://www.ncbi.nlm.nih.gov/Taxonomy
Newton AC, Fitt BD, Atkins SD et al (2010) Pathogenesis, parasitism and mutualism in the trophic space of microbe–plant interactions. Trends Microbiol 18:365–373
Normand P, Orso S, Cournoyer B et al (1996) Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int J Syst Bacteriol 46:1–9
Normand P, Lapierre P, Tisa LS et al (2007) Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res 17:7–15
Normander B, Christensen BB, Molin S, Kroer N (1998) Effect of bacterial distribution and activity on conjugal gene transfer on the phylloplane of the bush bean (Phaseolus vulgaris). Appl Environ Microbiol 64:1902–1909
Nunney L (2011) Homologous recomination and the invasion of a new plant host by the pathogenic bacterium, Xylella fastidiosa. Phytopathology 101:S130
Nunney L, Yuan X, Bromley RE, Stouthamer R (2012) Detecting genetic introgression: high levels of intersubspecific recombination found in Xylella fastidiosa in Brazil. Appl Environ Microbiol 78:4702–4714
Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4:701–712
Perrine FM, Hocart CH, Hynes MF, Rolfe BG (2005) Plasmid-associated genes in the model micro-symbiont Sinorhizobium meliloti 1021 affect the growth and development of young rice seedlings. Environ Microbiol 7:1826–1838
Persson T, Benson DR, Normand P et al (2011) Genome sequence of “Candidatus Frankia datiscae” Dg1, the uncultured microsymbiont from nitrogen-fixing root nodules of the dicot Datisca glomerata. J Bacteriol 193:7017–7018
Petriccione M, Di Cecco I, Arena S et al (2013) Proteomic changes in Actinidia chinensis shoot during systemic infection with a pandemic Pseudomonas syringae pv. actinidiae strain. J Proteomics 78:461–473
Piper KR, Beck von Bodman S, Farrand SK (1993) Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448–450
Preston GM (2004) Plant perception of plant growth-promoting Pseudomonas. Philos Trans R Soc Lond B Biol Sci 359:907–918
Preston GM, Bertrand N, Rainey PB (2001) Type III secretion in plant-growth-promoting Pseudomonas fluorescens SBW25. Mol Microbiol 41:999–1014
Price PW (1992) Evolutionary perspectives on host plants and their parasites. In: Andrews JH, Tommecup I (eds) Advances in plant pathology, vol 8. Academic, London, pp 1–30
Redenbach M, Scheel J, Schmidt U (2000) Chromosome topology and genome size of selected actinomycetes species. Antonie Leeuwenhoek 78:227–235
Remenant B, Coupat-Goutaland B, Guidot A et al (2010) Genomes of three tomato pathogens within the Ralstonia solanacearum species complex reveal significant evolutionary divergence. BMC Genomics 11:379
Ridley M (2004) Evolution. Blackwell, Malden, MA
Roberts AP, Mullany P (2009) A modular master on the move: the Tn916 family of mobile genetic elements. Trends Microbiol 17:251–258
Rogers EE, Stenger DC (2012) A conjugative 38 kb plasmid is present in multiple subspecies of Xylella fastidiosa. PLoS One 7:e52131
Ronchel MC, Ramos-Díaz MA, Ramos JL (2000) Retrotransfer of DNA in the rhizosphere. Environ Microbiol 2:319–323
Rosenberg C, Casse-Delbart F, Dusha I et al (1982) Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol 150:402–406
Ryan RP, Germaine K, Franks A et al (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9
Sarris PF, Trantas EA, Baltrus DA et al (2013) Comparative genomics of multiple strains of Pseudomonas cannabina pv. alisalensis, a potential model pathogen of both monocots and dicots. PLoS One 8:e59366
Schikora A, Garcia AV, Hirt H (2012) Plants as alternative hosts for Salmonella. Trends Plant Sci 17:245–249
Schneider DJ, Collmer AC (2010) Studying plant-pathogen interactions in the genomics era: beyond molecular Koch’s postulates to system biology. Annu Rev Phytopathol 48:457–479
Schneiker S, Keller M, Dröge M et al (2001) The genetic organization and evolution of the broad-host-range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res 29:5169–5181
Schuhegger R, Ihring A, Gantner S et al (2006) Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918
Schwaner NE, Kroer N (2001) Effect of plant species on the kinetics of conjugal transfer in the rhizosphere and relation to bacterial metabolic activity. Microb Ecol 42:458–465
Scortichini M (2005) The population structure of some plant pathogenic bacteria: an ecological and adaptive perspective. J Plant Pathol 87:5–12
Seitz P, Blokesch M (2013) Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol Rev 37:336–363
Sengeløv G, Kristensen KJ, Sørensen AH et al (2001) Effect of genomic location on horizontal transfer of a recombinant gene cassette between Pseudomonas strains in the rhizosphere and spermosphere of barley seedlings. Curr Microbiol 42:160–167
Sessitsch A, Kuffner M, Kidd P et al (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Silby MW, Winstanley G, Godfrey SA et al (2011) Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35:652–680
Slater SC, Goldman BS, Goodner B et al (2009) Genome sequences of three Agrobacterium biovars help elucidate the evolution of multichromosome genomes in bacteria. J Bacteriol 191:2501–2511
Slater S, Setubal JC, Goodner B et al (2013) Reconciliation of sequence data and updated annotation of the genome of Agrobacterium tumefaciens C58, and distribution of a linear chromosome in the genus Agrobacterium. Appl Environ Microbiol 79:1414–1417
Smillie C, Garcillán-Barcia MP, Francia MV et al (2010) Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452
Smit E, Wolters A, van Elsas JD (1998) Self-transmissible mercury resistance plasmids with gene-mobilizing capacity in soil bacterial populations: influence of wheat roots and mercury addition. Appl Environ Microbiol 64:1210–1219
Sobral BW, Honeycutt RJ, Atherly AG, McClelland M (1991) Electrophoretic separation of the three Rhizobium meliloti replicons. J Bacteriol 173:5173–5180
Stall RE, Loschke DC, Jones JB (1986) Linkage of copper resistance and avirulence loci on a self-transmissible plasmid in Xanthomonas campestris pv. vesicatoria. Phytopathology 76:240–243
Stokes HW, Gillings MR (2011) Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev 35:790–819
Studholme DJ, Wasukira A, Paszkiewicz K et al (2012) Draft genome sequences of Xanthomonas sacchari and two banana-associated xanthomonads reveal insights into the Xanthomonas group 1 clade. Genes 2:1050–1065
Sugawara M, Epstein B, Badgley B et al (2013) Comparative genomics of the core and accessory genomes of 48 Sinorhizobium strains comprising five genospecies. Genome Biol 14:R17
Sullivan JT, Ronson CW (1998) Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci U S A 95:5145–5149
Sullivan JT, Trzebiatowski JR, Cruickshank RW et al (2002) Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J Bacteriol 184:3086–3095
Sundin GW, McGhee GC, Foster GC et al (2004) Genetic analysis of the ubiquitous plasmid pEA29 and two new Erwinia amylovora plasmids. Acta Hortic 704:423–430
Suwanto A, Kaplan S (1989) Physical and genetic map** of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol 171:5840–5849
Taghavi S, Barac T, Borremans B et al (2005) Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl Environ Microbiol 71:8500–8505
Tan Z, Hurek T, Vinuesa P et al (2001) Specific detection of Bradyrhizobium and Rhizobium strains colonizing rice (Oryza sativa) roots by 16S-23S ribosomal DNA intergenic spacer-tergeted PCR. Appl Environ Microbiol 67:3655–3664
Tauch A, Schneiker S, Selbitschka W et al (2002) The complete nucleotide sequence and environmental distribution of the cryptic, conjugative, broad-host-range plasmid pIPO2 isolated from bacteria of the wheat rhizosphere. Microbiology 148:1637–1653
Teyssier-Cuvelle S, Mougel C, Nesme X (1999) Direct conjugal transfers of Ti plasmid to soil microflora. Mol Ecol 8:1273–1284
Teyssier-Cuvelle S, Oger P, Mougel C et al (2004) A highly selectable and highly transferable Ti plasmid to study conjugal host range and Ti plasmid dissemination in complex ecosystems. Microb Ecol 48:10–18
Troisfontaines P, Cornelis GR (2005) Type III secretion: more systems than you think. Physiology 20:326–339
Van der Auwera GA, Król JE, Suzuki H et al (2009) Plasmids captured in C. metallidurans CH34: defining the PromA family of broad-host-range plasmids. Antonie Leeuwenhoek 96:193–204
van Elsas JD, Gardener BB, Wolters AC, Smit E (1998) Isolation, characterization, and transfer of cryptic gene-mobilizing plasmids in the wheat rhizosphere. Appl Environ Microbiol 64:880–889
van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Van Sluys MA, Monteiro-Vitorello CB, Camargo LE et al (2002) Comparative genomic analysis of plant-associated bacteria. Annu Rev Phytopathol 40:169–189
Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB et al (2003) Comparative analysis of the complete genome sequences of Pierce’s disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol 185:1018–1026
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30:274–291
Vial L, Chapalain A, Groleau MC, Déziel E (2011) The various lifestyles of the Burkholderia cepacia complex species: a tribute to adaptation. Environ Microbiol 13:1–12
Viprey V, Del Greco A, Golinowski W et al (1998) Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol Microbiol 28:1381–1389
Walker V, Bruto M, Bellvert F et al (2013) Unexpected phytostimulatory behavior for Escherichia coli and Agrobacterium tumefaciens model strains. Mol Plant Microbe Interact 26:495–502
Wiedenbeck J, Cohan FM (2011) Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol Rev 35:957–976
Wing-yee L (2013) Discovery and genome analysis of the plant growth-promoting endophytic bacterium Enterobacter cloacae ENHK. PhD thesis. The University of Hong Kong, Pokfulam, Hong Kong
Wood DW, Setubal JC, Kaul R et al (2001) The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317–2323
Wozniak RA, Waldor MK (2010) Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 8:552–563
You M, Nishiguchi T, Saito A et al (2005) Expression of the nifH gene of a Herbaspirillum endophyte in wild rice species: daily rhythm during the light-dark cycle. Appl Environ Microbiol 71:8183–8190
Young JM, Kuykendall LD, Martínez-Romero E et al (2001) A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajude et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol 51:89–103
Young JPW, Crossman LC, Johnston AW et al (2006) The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol 7:R34
Zaneveld JR, Nemergut DR, Knight R (2008) Are all horizontal gene transfers created equal? Prospects for mechanism-based studies of HGT patterns. Microbiology 154:1–15
Acknowledgments
E.I. Katsy has been supported by the grant 12-04-00262-a from the Russian Foundation for Basic Research. We thank Dmitry N. Tychinin for improving our English.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Scortichini, M., Katsy, E.I. (2014). Common Themes and Specific Features in the Genomes of Phytopathogenic and Plant-Beneficial Bacteria. In: Katsy, E. (eds) Plasticity in Plant-Growth-Promoting and Phytopathogenic Bacteria. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9203-0_1
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9203-0_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9202-3
Online ISBN: 978-1-4614-9203-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)