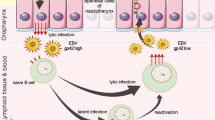
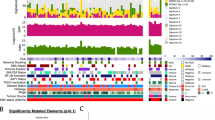
Abstract
Nasopharyngeal carcinoma (NPC) has a distinct geographic distribution and strong association with Epstein-Barr virus (EBV). Recent advances in molecular investigations and bioinformatics have disclosed critical genetic and epigenetic events in NPC. In this chapter, we will focus on important genetic and epigenetic alterations in NPC derived from EBV positive NPC cell lines and human tumoral tissues. Copy number losses on chromosomes 1p, 3p, 9p, 9q, 11q, 13q, 14q and 16q and recurrent gains on chromosome 1q, 3q, 8q, 12p and 12q were frequently observed in NPC. The roles of several important tumor suppressors (e.g., p16, RASSF1A) and oncogenes (e.g., CCND1, LTβR) have been delineated. However, potential critical cancer associated genes in other chromosomal regions remain to be identified. Frequent wide-spread methylation of cancer related genes is another common phenomenon in NPC and leads to alterations of multiple cellular pathways. The possible mechanisms of NPC tumorigenesis, in particular the roles of EBV latent gene products, have been suggested. There is also emerging information concerning the disruption of various signaling pathways including NF-κB signaling pathways in NPC. NPC serves as a fascinating model to understand the complex interaction among environmental, viral, and genetic factors in human tumorigenesis. Important genetic and epigenetic alterations in NPC are summarized in this chapter. Based on these observations, a hypothetical model of NPC tumorigenesis is proposed and serves as a platform for continuous refinement.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Ferlya J, Bray F, Pisani P et al. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0, IARC CancerBase No. 5: Lyon: IARC Press; 2001.
Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell 2004; 5:423–428.
Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 2002; 12:421–429.
Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 1986; 47:883–889.
Pathmanathan R, Prasad U, Sadler R et al. Clonal proliferations of cells infected with Epstein-Barr virus in pre-invasive lesions related to nasopharyngeal carcinoma. N Engl J Med 1995; 333:693–698.
Huang DP, Ho JH, Chan WK et al. Cytogenetics of undifferentiated nasopharyngeal carcinoma xenografts from southern Chinese. Int J Cancer 1989; 43:936–939.
Bernheim A, Rousselet G, Massaad L et al. Cytogenetic studies in three xenografted nasopharyngeal carcinomas. Cancer Genet Cytogenet 1993; 66:11–15.
Wong N, Hui AB, Fan B et al. Molecular cytogenetic characterization of nasopharyngeal carcinoma cell lines and xenografts by comparative genomic hybridization and spectral karyoty**. Cancer Genet Cytogenet 2003; 140:124–132.
Hui AB, Lo KW, Leung SF et al. Detection of recurrent chromosomal gains and losses in primary nasopharyngeal carcinoma by comparative genomic hybridisation. Int J Cancer 1999; 82:498–503.
Lo KW, Teo PM, Hui AB et al. High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res 2000; 60:3348–3353.
Chen YJ, Ko JY, Chen PJ et al. Chromosomal aberrations in nasopharyngeal carcinoma analyzed by comparative genomic hybridization. Genes Chromosomes Cancer 1999; 25:169–175.
Fang Y, Guan X, Guo Y et al. Analysis of genetic alterations in primary nasopharyngeal carcinoma by comparative genomic hybridization. Genes Chromosomes Cancer 2001; 30:254–260.
Hui AB, Or YY, Takano H et al. Array-based comparative genomic hybridization analysis identified cyclin Dl as a target oncogene at 11q13.3 in nasopharyngeal carcinoma. Cancer Res 2005; 65:8125–8133.
Huang Z, Desper R, Schaffer AA et al. Construction of tree models for pathogenesis of nasopharyngeal carcinoma. Genes Chromosomes Cancer 2004; 40:307–315.
Shih-Hsin Wu L. Construction of evolutionary tree models for nasopharyngeal carcinoma using comparative genomic hybridization data. Cancer Genet Cytogenet 2006; 168:105–108.
Chan AS, To KF, Lo KW et al. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res 2000; 60:5365–5370.
Chan AS, To KF, Lo KW et al. Frequent chromosome 9p losses in histologically normal nasopharyngeal epithelia from southern Chinese. Int J Cancer 2002; 102:300–303.
Or YY, Chung GT, To KF et al. Identification of a novel 12pl3.3 amplicon in nasopharyngeal carcinoma. JPathol 2010; 220:97–107.
Lo AK, Lo KW, Tsao SW et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia 2006; 8:173–180.
Stewart S, Dawson CW, Takada K et al. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc Natl Acad Sci U S A 2004; 101:15730–15735.
Thornburg NJ, Pathmanathan R, Raab-Traub N. Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res 2003; 63:8293–8301.
Eliopoulos AG, Caamano JH, Flavell J et al. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52 via an IKKgamma/NEMO-independent signalling pathway. Oncogene 2003; 22:7557–7569.
Or YY, Hui AB, Tarn KY et al. Characterization of chromosome 3q and 12q amplicons in nasopharyngeal carcinoma cell lines. Int J Oncol 2005; 26:49–56.
Samuels Y, Wang Z, Bardelli A et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304:554.
Lee JW, Soung YH, Kim SY et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene 2005; 24:1477–1480.
Or YY, Hui AB, To KF et al. PIK3CA mutations in nasopharyngeal carcinoma. Int J Cancer 2006; 118:1065–1067.
van Lohuizen M, Verbeek S, Scheijen B et al. Identification of co-operating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 1991; 65:737–752.
Haupt Y, Alexander WS, Barri G et al. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 1991; 65:753–763.
Song LB, Zeng MS, Liao WT et al. Bmi-1 is anovel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 2006; 66:6225–6232.
Li HM, Man C, ** Y et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer 2006; 119:1567–1576.
Alajez NM, Shi W, Hui AB et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a,miR-101, and miR-98. Cell Death Dis. l:e85,2010.
Lu JJ, Chen CL, Hsu TY et al. Expression of Epstein-Barr virus latent membrane protein 1 and B-cell leukemia-lymphoma 2 gene in nasopharyngeal carcinoma tissues. J Microbiol Immunol Infect 2002; 35:136–140.
Yip KW, Shi W, Pintilie M et al. Prognostic significance of the Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal cancer. Clin Cancer Res 2006; 12:5726–5732.
Yu Y, Dong W, Li X et al. Significance of c-Myc and Bcl-2 protein expression in nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2003; 129:1322–1326.
Tsujimoto Y, Gorham J, Cossman J et al. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 1985; 229:1390–1393.
Lu JJ, Chen JY, Hsu TY et al. Co-operative interaction between Bcl-2 and Epstein-Barr virus latent membrane protein 1 in the growth transformation of human epithelial cells. J Gen Virol 1997; 78 (Pt 11):2975–2985.
Huang DP, Lo KW, van Hasselt CA et al. A region of homozygous deletion on chromosome 9p21-22 in primary nasopharyngeal carcinoma. Cancer Res 1994; 54:4003–4006.
Lo KW, Huang DP, Lau KM. p16 gene alterations in nasopharyngeal carcinoma. Cancer Res 1995; 55:2039–2043.
Lo KW, Cheung ST, Leung SF et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res 1996; 56:2721–2725.
Kwong J, Lo KW, To KF et al. Promoter hypermethylation of multiple genes in nasopharyngeal carcinoma. Clin Cancer Res 2002; 8:131–137.
Kamb A, Gruis NA, Weaver-Feldhaus J et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994; 264:436–440.
Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell 2006; 127:265–275.
Wang GL, Lo KW, Tsang KS et al. Inhibiting tumorigenic potential by restoration of pl6 in nasopharyngeal carcinoma. Br J Cancer 1999; 81:1122–1126.
Spruck CH 3rd, Tsai YC, Huang DP et al. Absence of p53 gene mutations in primary nasopharyngeal carcinomas. Cancer Res 1992; 52:4787–4790.
Sun Y, Hegamyer G, Cheng YJ et al. An infrequent point mutation of the p53 gene in human nasopharyngeal carcinoma. Proc Natl Acad Sci U S A 1992; 89:6516–6520.
Effert P, McCoy R, Abdel-Hamid M et al. Alterations of the p53 gene in nasopharyngeal carcinoma. J Virol 1992; 66:3768–3775.
Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene 2002; 21:6915–6935.
Huang DP, Lo KW, Choi PH et al. Loss of heterozygosity on the short arm of chromosome 3 in nasopharyngeal carcinoma. Cancer Genet Cytogenet 1991; 54:91–99.
Lo KW, Tsao SW, Leung SF et al. Detailed deletion map** on the short arm of chromosome 3 in nasopharyngeal carcinomas. Int J Oncol 1994; 4:1359–1364.
Lo KW, Kwong J, Hui AB et al. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res 2001; 61:3877–3881.
Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res 2000; 60:6116–6133.
Dammann R, Li C, Yoon JH et al. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 2000; 25:315–319.
Dammann R, Schagdarsurengin U, Seidel C et al. The tumor suppressor RASSF1A inhuman carcinogenesis: an update. Histol Histopathol 2005; 20:645–663.
Burbee DG, Forgacs E, Zochbauer-Muller S et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst 2001; 93:691–699.
Dreijerink K, Braga E, Kuzmin I et al. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc Natl Acad Sci U S A 2001; 98:7504–7509.
Schagdarsurengin U, Wilkens L, Steinemann D et al. Frequent epigenetic inactivation of the RASSF1A gene in hepatocellular carcinoma. Oncogene 2003; 22:1866–1871.
Lusher ME, Lindsey JC, Latif F et al. Biallelic epigenetic inactivation of the RASSF1A tumor suppressor gene in medulloblastoma development. Cancer Res 2002; 62:5906–5911.
Chow LS, Lo KW, Kwong J et al. RASSFIA is a target tumor suppressor from 3p21.3 in nasopharyngeal carcinoma. Int J Cancer 2004; 109:839–847.
Vos MD, Ellis CA, Bell A et al. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J Biol Chem 2000; 275:35669–35672.
Rabizadeh S, Xavier RJ, Ishiguro K et al. The scaffold protein CNK1 interacts with the tumor suppressor RASSFIA and augments RASSF1A-induced cell death. J Biol Chem 2004; 279:29247–29254.
Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res 2005; 65:3497–3508.
Shivakumar L, Minna J, Sakamaki T et al. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol 2002; 22:4309–4318.
Whang YM, Kim YH, Kim JS et al. RASSF1A suppresses the c-Jun-NH2-kinase pathway and inhibits cell cycle progression. Cancer Res 2005; 65:3682–3690.
Chow LS, Lam CW, Chan SY et al. Identification of RASSF1A modulated genes in nasopharyngeal carcinoma. Oncogene 2006; 25:310–316.
Liu L, Tommasi S, Lee DH et al. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 2003; 22:8125–8136.
Song MS, Chang JS, Song SJ et al. The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem 2005; 280:3920–3927.
Song MS, Song SJ, Ayad NG et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 2004; 6:129–137.
Mathe E. RASSF1A, the new guardian of mitosis. Nat Genet 2004; 36:117–118.
Liu L, Vo A, McKeehan WL. Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5. Cancer Res 2005; 65:1830–1838.
Dallol A, Cooper WN, AI-Mulla F et al. Depletion of the Ras association domain family 1, isoform A-associated novel microtubule-associated protein, C19ORF5/MAP1S, causes mitotic abnormalities. Cancer Res 2007; 67:492–500.
Guo C, Tommasi S, Liu L et al. RASSFIA is part of a complex similar to the drosophila hippo/salvador/ lats tumor-suppressor network. Curr Biol 2007; 17:700–705.
Man C, Rosa J, Lee LT et al. Latent membrane protein 1 suppresses RASSFIA expression, disrupts microtubule structures and induces chromosomal aberrations in human epithelial cells. Oncogene 2006.
Chow C, Wong N, Pagano M et al. Regulation of APC/C(Cdc20) activity by RASSF1A-APC/C(Cdc20) circuitry. Oncogene. 2011 in print.
Tommasi S, Dammann R, Zhang Z et al. Tumor susceptibility of Rassfla knockout mice. Cancer Res 2005; 65:92–98.
Chow LS, Lo KW, Kwong J et al. Aberrant methylation of RASSF4/AD037 in nasopharyngeal carcinoma. Oncol Rep 2004; 12:781–787.
Zhang Z, Sun D, Van do N et al. Inactivation of RASSF2A by promoter methylation correlates with lymph node metastasis in nasopharyngeal carcinoma. Int J Cancer 2007; 120:32–38.
Yau WL, Lung HL, Zabarovsky ER et al. Functional studies of the chromosome 3p21.3 candidate tumor suppressor gene BLU/ZMYND10 in nasopharyngeal carcinoma. Int J Cancer 2006; 119:2821–2826.
Liu XQ, Chen HK, Zhang XS et al. Alterations of BLU, a candidate tumor suppressor gene on chromosome 3p21.3, in human nasopharyngeal carcinoma. Int J Cancer 2003; 106:60–65.
Qiu GH, Tan LK, Loh KS et al. The candidate tumor suppressor gene BLU, located at the commonly deleted region 3p21.3, is an E2F-regulated, stress-responsive gene and inactivated by both epigenetic and genetic mechanisms in nasopharyngeal carcinoma. Oncogene 2004; 23:4793–4806.
Agathanggelou A, Dallol A, Zochbauer-Muller S et al. Epigenetic inactivation of the candidate 3p21.3 suppressor gene BLU in human cancers. Oncogene 2003; 22:1580–1588.
Kwong J, Chow LS, Wong AY et al. Epigenetic inactivation of the deleted in lung and esophageal cancer 1 gene in nasopharyngeal carcinoma. Genes Chromosomes Cancer 2007; 46:171–180.
Kwong J, Lee JY, Wong KK et al. Candidate tumor-suppressor gene DLEC1 is frequently downregulated by promoter hypermethylation and histone hypoacetylation in human epithelial ovarian cancer. Neoplasia 2006; 8:268–278.
Kuramochi M, Fukuhara H, Nobukuni T et al. TSLC1 is a tumor-suppressor gene in human nonsmall-cell lung cancer. Nat Genet 2001; 27:427–430.
Masuda M, Yageta M, Fukuhara H et al. The tumor suppressor protein TSLC1 is involved in cell-cell adhesion. J Biol Chem 2002; 277: 31014–31019.
Shingai T, Ikeda W, Kakunaga S et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/ TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem 2003; 278:35421–35427.
Yageta M, Kuramochi M, Masuda M et al. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res 2002; 62:5129–5133.
Masuda M, Kikuchi S, Maruyama T et al. Tumor suppressor in lung cancer (TSLC)1 suppresses epithelial cell scattering and tubulogenesis. J Biol Chem 2005; 280:42164–42171.
Hui AB, Lo KW, Kwong J et al. Epigenetic inactivation of TSLC1 gene in nasopharyngeal carcinoma. Mol Carcinog 2003; 38:170–178.
Lung HL, Leung Cheung AK, **e D et al. TSLC1 is a tumor suppressor gene associated with metastasis in nasopharyngeal carcinoma. Cancer Res 2006; 66:9385–9392.
Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta 2006; 1763:991–999.
Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 2006; 20:1045–1054.
Abeysinghe HR, Li LQ, Guckert NL et al. THY-1 induction is associated with up-regulation of fibronectin and thrombospondin-1 in human ovarian cancer. Cancer Genet Cytogenet 2005; 161:151–158.
Lung HL, Bangarusamy DK, **e D et al. THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene 2005; 24:6525–6532.
Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007; 128:683–692.
Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 2006; 6:107–116.
Ting AH, McGarvey KM, Baylin SB. The cancer epigenome—components and functional correlates. Genes Dev 2006; 20:3215–3231.
Kwong J, Lo KW, Chow LS et al. Epigenetic silencing of cellular retinol-binding proteins in nasopharyngeal carcinoma. Neoplasia 2005; 7:67–74.
Kwong J, Lo KW, Chow LS et al. Silencing of the retinoid response gene TIG 1 by promoter hypermethylation in nasopharyngeal carcinoma. Int J Cancer 2005; 113:386–392.
Ying J, Li H, Seng TJ et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene 2006; 25:1070–1080.
Tsao SW, Liu Y, Wang X et al. The association of E-cadherin expression and the methylation status of the E-cadherin gene in nasopharyngeal carcinoma cells. Eur J Cancer 2003; 39:524–531.
Sun D, Zhang Z, Van do N et al. Aberrant methylation of CDH13 gene in nasopharyngeal carcinoma could serve as a potential diagnostic biomarker. Oral Oncol 2007; 43:82–87.
Lo KW, Tsang YS, Kwong J et al. Promoter hypermethylation of the EDNRB gene in nasopharyngeal carcinoma. Int J Cancer 2002; 98:651–655.
Wong TS, Kwong DL, Sham JS et al. Promoter hypermethylation of high-in-normal 1 gene in primary nasopharyngeal carcinoma. Clin Cancer Res 2003; 9:3042–3046.
Chan SL, Cui Y, van Hasselt A et al. The tumor suppressor Wnt inhibitory factor 1 is frequently methylated in nasopharyngeal and esophageal carcinomas. Lab Invest 2007.
Lee KY, Geng H, Ng KM et al. Epigenetic disruption of interferon-gamma response through silencing the tumor suppressor interferon regulatory factor 8 in nasopharyngeal, esophageal and multiple other carcinomas. Oncogene 2008; 27:5267–5276.
Li L, Tao Q, ** H et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res 2010; 16:2949–2958.
Ying J, Srivastava G, Hsieh WS et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res 2005; 11:6442–6449.
** H, Wang X, Ying J et al. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci U S A 2007; 104:12353–12358.
Tong JH, Ng DC, Chau SL et al. Putative tumour-suppressor gene DAB2 is frequently down regulated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer 2010; 10:253.
Cheung HW, Ching YP, Nicholls JM et al. Epigenetic inactivation of CHFR in nasopharyngeal carcinoma through promoter methylation. Mol Carcinog 2005; 43:237–245.
Chang HW, Chan A, Kwong DL et al. Detection of hypermethylated RIZ1 gene in primary tumor, mouth, and throat rinsing fluid, nasopharyngeal swab, and peripheral blood of nasopharyngeal carcinoma patient. Clin Cancer Res 2003; 9:1033–1038.
Kang GH, Lee S, Kim WH et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol 2002; 160:787–794.
Minarovits J. Epigenotypes of latent herpesvirus genomes. Curr Top Microbiol Immunol 2006; 310:61–80.
Li H, Minarovits J. Host cell-dependent expression of latent Epstein-Barr virus genomes: regulation by DNA methylation. Adv Cancer Res 2003; 89:133–156.
Tsai CL, Li HP, Lu YJ et al. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH(2)-terminal kinase signaling. Cancer Res 2006; 66:11668–11676.
Tsai CN, Tsai CL, Tse KP et al. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci U S A 2002; 99:10084–10089.
Dutton A, Woodman CB, Chukwuma MB et al. Bmi-1 is induced by the Epstein-Barr virus oncogene LMP1 and regulates the expression of viral target genes in Hodgkin lymphoma cells. Blood 2007; 109:2597–2603.
Ohm JE, McGarvey KM, Yu X et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet 2007; 39:237–242.
Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4:757–768.
Young LS, Murray PG. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene 2003; 22:5108–5121.
Tsao SW, Tramoutanis G, Dawson CW et al. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol 2002; 12:473–487.
Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol 2002; 12:431–441.
Shi W, Bastianutto C, Li A et al. Multiple dysregulated pathways in nasopharyngeal carcinoma revealed by gene expression profiling. Int J Cancer 2006; 119:2467–2475.
Ruco LP, Stoppacciaro A, Uccini S et al. Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in undifferentiated nasopharyngeal carcinoma (lymphoepithelioma) and in malignant epithelial tumors. Hum Pathol 1994; 25:924–928.
Codd JD, Salisbury JR, Packham G et al. A20 RNA expression is associated with undifferentiated nasopharyngeal carcinoma and poorly differentiated head and neck squamous cell carcinoma. J Pathol 1999; 187:549–555.
Ma BB, Poon TC, To KF et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma—a prospective study. Head Neck 2003; 25:864–872.
Wang N, Wu QL, Fang Y et al. Expression of chemokine receptor CXCR4 in nasopharyngeal carcinoma: pattern of expression and correlation with clinical outcome. J Transi Med 2005; 3:26.
Hu J, Deng X, Bian X et al. The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res 2005; 11:4658–4665.
Brown HJ, Song MJ, Deng H et al. NF-kappaB inhibits gammaherpesvirus lytic replication. J Virol 2003; 77:8532–8540.
Man CH, Lun SWM, Hui JWY et al. Inhibition of NOTCH3 signaling significantly enhances sensitivity to cisplatin in EBV-associated nasopharyngeal carcinoma. J Path. 2011, in print.
Morrison JA, Gulley ML, Pathmanathan R et al. Differential signaling pathways are activated in the Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res 2004; 64:5251–5260.
Sheu LF, Chen A, Meng CL et al. Analysis of Bcl-2 expression in normal, inflamed, dysplastic nasopharyngeal epithelia, and nasopharyngeal carcinoma: association with p53 expression. Hum Pathol 1997; 28:556–562.
Cheung FM, Pang SW, Yau TK et al. Nasopharyngeal intraepithelial lesion: latent Epstein-Barr virus infection with malignant potential. Histopathology 2004; 45:171–179.
Chang JT, Liao CT, Jung SM et al. Telomerase activity is frequently found in metaplastic and malignant human nasopharyngeal tissues. Br J Cancer 2000; 82:1946–1951.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Landes Bioscience and Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Lo, KW., Chung, G.TY., To, KF. (2013). Acquired Genetic and Epigenetic Alterations in Nasopharyngeal Carcinoma. In: Busson, P. (eds) Nasopharyngeal Carcinoma. Advances in Experimental Medicine and Biology, vol 778. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5947-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5947-7_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5946-0
Online ISBN: 978-1-4614-5947-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)