Abstract
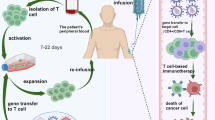
Human T lymphocytes that transgenically express a chimeric antigen receptor (CAR) have proven efficacy and safety in gene- and cell-based immunotherapy of certain hematological cancers. Appropriate gene vectors and methods of genetic engineering are required for therapeutic cell products to be biologically potent and their manufacturing to be economically viable. Transposon-based gene transfer satisfies these needs, and is currently being evaluated in clinical trials. In this protocol we describe the basic Slee** Beauty (SB) transposon vector components required for stable gene integration in human cells, with special emphasis on minicircle DNA vectors and the use of synthetic mRNA. We provide a protocol for functional validation of the vector components in cultured human cell lines on the basis of fluorescent reporter gene expression. Finally, we provide a protocol for CAR-T cell engineering and describe assays that address transgene expression, biological potency and genomic vector copy numbers in polyclonal cell populations. Because transposons allow virus-free gene transfer with naked nucleic acids, the protocol can be adopted by any laboratory equipped with biological safety level S1 facilities.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mullard A (2017) FDA approves first CAR T therapy. Nat Rev Drug Discov 16(10):669
van der Loo JC, Wright JF (2016) Progress and challenges in viral vector manufacturing. Hum Mol Genet 25(R1):R42–R52
Ivics Z et al (1997) Molecular reconstruction of slee** beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91(4):501–510
Amberger M, Ivics Z (2020) Latest advances for the slee** beauty transposon system: 23 years of insomnia but prettier than ever. BioEssays 42(11):e2000136
Ochmann MT, Ivics Z (2021) Jum** ahead with slee** beauty: mechanistic insights into cut-and-paste transposition. Viruses 13(1):76
Ivics Z et al (2009) Transposon-mediated genome manipulation in vertebrates. Nat Methods 6(6):415–422
Hackett PB, Largaespada DA, Cooper LJN (2010) A transposon and transposase system for human application. Mol Ther 18(4):674–683
Kebriaei P et al (2017) Gene therapy with the slee** beauty transposon system. Trends Genet 33(11):852–870
Hackett PB et al (2005) Slee** beauty transposon-mediated gene therapy for prolonged expression. Adv Genet 54:189–232
Zayed H et al (2004) Development of hyperactive slee** beauty transposon vectors by mutational analysis. Mol Ther 9(2):292–304
Hackett PB et al (2013) Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl Res 161(4):265–283
Hudecek M, Ivics Z (2018) Non-viral therapeutic cell engineering with the slee** beauty transposon system. Curr Opin Genet Dev 52:100–108
Singh H et al (2013) Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using slee** beauty system and artificial antigen presenting cells. PLoS One 8(5):e64138
Singh H et al (2014) A new approach to gene therapy using slee** beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev 257(1):181–190
Singh H et al (2008) Redirecting specificity of T-cell populations for CD19 using the slee** beauty system. Cancer Res 68(8):2961–2971
Singh H et al (2015) Manufacture of T cells using the slee** beauty system to enforce expression of a CD19-specific chimeric antigen receptor. Cancer Gene Ther 22(2):95–100
Chicaybam L et al (2020) Transposon-mediated generation of CAR-T cells shows efficient anti B-cell leukemia response after ex vivo expansion. Gene Ther 27(1–2):85–95
Clauss J et al (2018) Efficient non-viral T-cell engineering by slee** beauty minicircles diminishing DNA toxicity and miRNAs silencing the endogenous T-cell receptors. Hum Gene Ther 29(5):569–584
Kebriaei P et al (2016) Phase I trials using slee** beauty to generate CD19-specific CAR T cells. J Clin Invest 126(9):3363–3376
Magnani CF et al (2020) Slee** beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest 130(11):6021–6033
Mayrhofer P, Schleef M, Jechlinger W (2009) Use of minicircle plasmids for gene therapy. Methods Mol Biol 542:87–104
Holstein M et al (2018) Efficient non-viral gene delivery into human hematopoietic stem cells by minicircle slee** beauty transposon vectors. Mol Ther 26(4):1137–1153
Monjezi R et al (2017) Enhanced CAR T-cell engineering using non-viral slee** beauty transposition from minicircle vectors. Leukemia 31(1):186–194
Prommersberger S et al (2021) CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free slee** beauty gene transfer to treat multiple myeloma. Gene Ther 28(9):560–571. https://doi.org/10.1038/s41434-021-00254-w
Querques I et al (2019) A highly soluble slee** beauty transposase improves control of gene insertion. Nat Biotechnol 37(12):1502–1512
Cui Z et al (2002) Structure-function analysis of the inverted terminal repeats of the slee** beauty transposon. J Mol Biol 318(5):1221–1235
Mates L et al (2009) Molecular evolution of a novel hyperactive slee** beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet 41(6):753–761
Wang X et al (2011) A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118(5):1255–1263
Brown CE et al (2005) Biophotonic cytotoxicity assay for high-throughput screening of cytolytic killing. J Immunol Methods 297(1–2):39–52
Wang Y et al (2017) Regulated complex assembly safeguards the fidelity of slee** beauty transposition. Nucleic Acids Res 45(1):311–326
Ivics Z et al (2014) Germline transgenesis in rodents by pronuclear microinjection of slee** beauty transposons. Nat Protoc 9(4):773–793
Kowarz E, Loscher D, Marschalek R (2015) Optimized slee** beauty transposons rapidly generate stable transgenic cell lines. Biotechnol J 10(4):647–653
Huang X et al (2008) Slee** beauty transposon-mediated engineering of human primary T cells for therapy of CD19+ lymphoid malignancies. Mol Ther 16(3):580–589
Acknowledgments
M.H. and Z.I. is receiving funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 754658 (CARAMBA). S.P. and M.H. are supported by the patient advocacy group ‘Hilfe im Kampf gegen den Krebs e.V.’, Würzburg, Germany and ‘Forschung hilft’—Stiftung zur Förderung der Krebsforschung an der Universität Würzburg. Further, the authors are supported by the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 853988 (imSAVAR to M.H.) and No. 945393 (T2EVOLVE to M.H. and Z.I.). Some of the figures were prepared with the help of Biorender.com.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Prommersberger, S. et al. (2022). Generation of CAR-T Cells with Slee** Beauty Transposon Gene Transfer. In: Walther, W. (eds) Gene Therapy of Cancer. Methods in Molecular Biology, vol 2521. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2441-8_3
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2441-8_3
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2440-1
Online ISBN: 978-1-0716-2441-8
eBook Packages: Springer Protocols