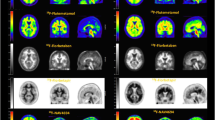
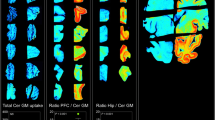
Abstract
Alzheimer’s disease (AD), an irreversible, progressive neurodegenerative disorder clinically characterized by memory loss and cognitive decline, is the leading cause of dementia in the elderly, leading invariably to death within 7–10 years after diagnosis. In vivo amyloid imaging with positron emission tomography (PET) is allowing new insights into β-amyloid (Aβ) deposition in the brain, facilitating research into the causes, diagnosis, and future treatment of dementias, where Aβ may play a role. Non-invasive quantification of Aβ burden in the brain with PET has proven useful in the early and differential diagnosis of dementias, showing significantly higher retention in grey matter of AD patients when compared with healthy controls (HC) or patients with frontotemporal lobe degeneration (FTLD). With the advent of new therapeutic strategies aimed at reducing Aβ burden in the brain to potentially prevent or delay functional and irreversible cognitive loss, there is increased interest in develo** agents that allow assessment of Aβ burden in vivo. A key aspect for Aβ burden quantification is the application of compartmental or graphical analyses to the kinetic data in order to obtain quantitative and reproducible statements that allow comparison with other nosological groups, correlation with cognitive or biological parameters, and selection, monitoring, and follow-up of individuals in disease modifying therapeutic trials. It is also a necessary step in the validation of simplified approaches that could be applied in routine clinical settings. With the availability of novel amyloid imaging agents radiolabeled with either 11C (half-life 20 min) or 18F (half-life 110 min), a description of different image acquisition approaches is provided.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Masters, C. L., Cappai, R., Barnham, K. J., and Villemagne, V. L. (2006) Molecular mechanisms for Alzheimer’s disease: implications for neuroimaging and therapeutics. J. Neurochem. 97(6), 1700–25.
Bennett, D. A. (2000) Part I. Epidemiology and public health impact of Alzheimer’s disease. Dis. Mon. 46(10), 657–65.
Johnson, N., Davis, T., and Bosanquet, N. (2000) The epidemic of Alzheimer’s disease. How can we manage the costs? Pharmacoeconomics 18(3), 215–23.
Schneider, J., Murray, J., Banerjee, S., and Mann, A. (1999) EUROCARE: a cross-national study of co-resident spouse carers for people with Alzheimer’s disease: I – Factors associated with carer burden. Int. J. Geriatr. Psychiatry 14(8), 651–61.
Selkoe, D. J. (2002) Alzheimer’s disease is a synaptic failure. Science 298(5594), 789–91.
Masters, C. L. (2005) Neuropathology of Alzheimer’s disease. In Dementia, Burns, A., O’Brien, J., and Ames, D., (Eds.), 3rd ed. Hodder Arnold, London, pp. 393–407.
Masters, C. L. and Beyreuther, K. (2005) The neuropathology of Alzheimer’s disease in the year 2005. In Neurodegenerative diseases: neurobiology, pathogenesis and therapeutics, Beal, M. F., Lang, A. E., and Ludolph, A. C., (Eds.). Cambridge University Press, Cambridge, MA, pp. 433–40.
Jellinger, K. (1990) Morphology of Alzheimer disease and related disorders. In Alzheimer disease: epidemiology, neuropathology, neurochemistry, and clinics, Maurer, K., Riederer, P., and Beckmann, H., (Eds.). Springer, Berlin, pp. 61–77.
Michaelis, M. L., Dobrowsky, R. T., and Li, G. (2002) Tau neurofibrillary pathology and microtubule stability. J. Mol. Neurosci. 19(3), 289–93.
Jellinger, K. A. and Bancher, C. (1998) Neuropathology of Alzheimer’s disease: a critical update. J. Neural. Transm. Suppl. 54, 77–95.
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., and Beyreuther, K. (1985) Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc. Natl. Acad. Sci. USA 82(12), 4245–9.
Cappai, R. and White, A. R. (1999) Amyloid beta. Int. J. Biochem. Cell Biol. 31(9), 885–9.
Villemagne, V. L., Cappai, R., Barnham, K. J., Cherny, R., Opazo, C., Novakovic, K. E., Rowe, C. C., and Masters, C. L. (2006) The Abeta centric pathway of Alzheimer’s disease. In Abeta peptide and Alzheimer’s disease, Barrow, C. J. and Small, B. J., (Eds.). Springer, London, pp. 5–32.
McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Beyreuther, K., Bush, A. I., and Masters, C. L. (1999) Soluble pool of Aß amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46(6), 860–6.
Naslund, J., Haroutunian, V., Mohs, R., Davis, K. L., Davies, P., Greengard, P., and Buxbaum, J. D. (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283(12), 1571–7.
Lue, L. F., Kuo, Y. M., Roher, A. E., Brachova, L., Shen, Y., Sue, L., Beach, T., Kurth, J. H., Rydel, R. E., and Rogers, J. (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 155(3), 853–62.
Wang, J., Dickson, D. W., Trojanowski, J. Q., and Lee, V. M. (1999) The levels of soluble versus insoluble brain Aß distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 158(2), 328–37.
Walsh, D. M., Klyubin, I., Shankar, G. M., Townsend, M., Fadeeva, J. V., Betts, V., Podlisny, M. B., Cleary, J. P., Ashe, K. H., Rowan, M. J., et al. (2005) The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 33(Pt 5), 1087–90.
Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J., and Selkoe, D. J. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416(6880), 535–9.
Petersen, R. C., Stevens, J. C., Ganguli, M., Tangalos, E. G., Cummings, J. L., and DeKosky, S. T. (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review)-report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56(9), 1133–42.
Petersen, R. C. (2000) Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia 15(3), 93–101.
Petersen, R. C. and Knopman, D. S. (2006) MCI is a clinically useful concept. Int. Psychogeriatr. 18(3), 394–402; discussion 409–314.
Selkoe, D. J. (1991) The molecular pathology of Alzheimer’s disease. Neuron 6(4), 487–98.
Hardy, J. A. and Higgins, G. A. (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054), 184–5.
Robinson, S. R. and Bishop, G. M. (2002) The search for an amyloid solution. Science 298(5595), 962–4; author reply 962–964.
Hardy, J. (1997) Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 20(4), 154–9.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984) Clinical Diagnosis of Alzheimer’s Disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–44.
Masters, C. L. and Beyreuther, K. (2006) Alzheimer’s centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain 129(Pt 11), 2823–39.
Schenk, D., Hagen, M., and Seubert, P. (2004) Current progress in beta-amyloid immunotherapy. Curr. Opin. Immunol. 16(5), 599–606.
Dubois, B., Feldman, H. H., Jacova, C., Dekosky, S. T., Barberger-Gateau, P., Cummings, J., Delacourte, A., Galasko, D., Gauthier, S., Jicha, G., et al. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6(8), 734–46.
Selkoe, D. J. (2000) The early diagnosis of Alzheimer’s disease. In The pathophysiology of Alzheimer’s disease, Scinto, L. F. M. and Daffner, K. R., (Eds.). Humana, Totowa, NJ, pp. 83–104.
Villemagne, V. L., Ng, S., Cappai, R., Barnham, K. J., Fodero-Tavoletti, M. T., Rowe, C. C., and Masters, C. L. (2006) La Lunga Attesa: towards a molecular approach to neuroimaging and therapeutics in alzheimer’s disease. Neuroradiol. J. 19, 51–75.
Mathis, C. A., Lopresti, B. J., and Klunk, W. E. (2007) Impact of amyloid imaging on drug development in Alzheimer’s disease. Nucl. Med. Biol. 34(7), 809–22.
Sair, H. I., Doraiswamy, P. M., and Petrella, J. R. (2004) In vivo amyloid imaging in Alzheimer’s disease. Neuroradiology 46(2), 93–104.
Villemagne, V. L., Rowe, C. C., Macfarlane, S., Novakovic, K. E., and Masters, C. L. (2005) Imaginem oblivionis: the prospects of neuroimaging for early detection of Alzheimer’s disease. J. Clin. Neurosci. 12(3), 221–30.
Ono, M., Wilson, A., Nobrega, J., Westaway, D., Verhoeff, P., Zhuang, Z. P., Kung, M. P., and Kung, H. F. (2003) 11C-labeled stilbene derivatives as Abeta-aggregate-specific PET imaging agents for Alzheimer’s disease. Nucl. Med. Biol. 30(6), 565–71.
Kung, M. P., Skovronsky, D. M., Hou, C., Zhuang, Z. P., Gur, T. L., Zhang, B., Trojanowski, J. Q., Lee, V. M., and Kung, H. F. (2003) Detection of amyloid plaques by radioligands for Abeta40 and Abeta42: potential imaging agents in Alzheimer’s patients. J. Mol. Neurosci. 20(1), 15–24.
Mathis, C. A., Bacskai, B. J., Kajdasz, S. T., McLellan, M. E., Frosch, M. P., Hyman, B. T., Holt, D. P., Wang, Y., Huang, G. F., Debnath, M. L., et al. (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg. Med. Chem. Lett. 12(3), 295–8.
Zhang, W., Oya, S., Kung, M. P., Hou, C., Maier, D. L., and Kung, H. F. (2005) F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl. Med. Biol. 32(8), 799–809.
Kudo, Y. (2006) Development of amyloid imaging PET probes for an early diagnosis of Alzheimer’s disease. Minim. Invasive Ther. Allied Technol. 15(4), 209–13.
Mathis, C. A., Wang, Y., Holt, D. P., Huang, G. F., Debnath, M. L., and Klunk, W. E. (2003) Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J. Med. Chem. 46(13), 2740–54.
Klunk, W. E., Wang, Y., Huang, G. F., Debnath, M. L., Holt, D. P., Shao, L., Hamilton, R. L., Ikonomovic, M. D., DeKosky, S. T., and Mathis, C. A. (2003) The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J. Neurosci. 23(6), 2086–92.
Kung, M. P., Hou, C., Zhuang, Z. P., Skovronsky, D., and Kung, H. F. (2004) Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer’s disease. Brain Res. 1025(1–2), 98–105.
Maezawa, I., Hong, H. S., Liu, R., Wu, C. Y., Cheng, R. H., Kung, M. P., Kung, H. F., Lam, K. S., Oddo, S., Laferla, F. M., et al. (2008) Congo red and thioflavin-T analogs detect Abeta oligomers. J. Neurochem. 104(2), 457–68.
Agdeppa, E. D., Kepe, V., Petri, A., Satyamurthy, N., Liu, J., Huang, S. C., Small, G. W., Cole, G. M., and Barrio, J. R. (2003)In vitro detection of (S)-naproxen and ibuprofen binding to plaques in the Alzheimer’s brain using the positron emission tomography molecular imaging probe 2-(1-[6-[(2-[(18)F]fluoroethyl)(methyl)amino]-2-naphthyl]ethylidene)malononitrile. Neuroscience 117(3), 723–30.
Barrio, J. R., Huang, S. C., Cole, G., Satyamurthy, N., Petric, A., Phelps, M. E., and Small, G. (1999) PET imaging of tangles and plaques in Alzheimer disease with a highly lipophilic probe. J. Label. Compd. Radiopharm. 42, S194–5.
Shoghi-Jadid, K., Small, G. W., Agdeppa, E. D., Kepe, V., Ercoli, L. M., Siddarth, P., Read, S., Satyamurthy, N., Petric, A., Huang, S. C., et al. (2002) Localisation of neurofibrillary tangles and ß-amyloid plaques in the brains of living patients with Alzheimer’s disease. Am. J. Geriatr. Psychiatry 10(1), 24–35.
Small, G. W., Agdeppa, E. D., Kepe, V., Satyamurthy, N., Huang, S. C., and Barrio, J. R. (2002) In vivo brain imaging of tangle burden in humans. J. Mol. Neurosci. 19(3), 323–7.
Small, G. W., Kepe, V., Ercoli, L. M., Siddarth, P., Bookheimer, S. Y., Miller, K. J., Lavretsky, H., Burggren, A. C., Cole, G. M., Vinters, H. V., et al. (2006) PET of brain amyloid and tau in mild cognitive impairment. N. Engl. J. Med. 355(25), 2652–63.
Lee, V. M. (2002) Related Amyloid binding ligands as Alzheimer’s disease therapies. Neurobiol. Aging 23(6), 1039–42.
Marshall, J. R., Stimson, E. R., Ghilardi, J. R., Vinters, H. V., Mantyh, P. W., and Maggio, J. E. (2002) Noninvasive imaging of peripherally injected Alzheimer’s disease type synthetic A beta amyloid in vivo. Bioconjug. Chem. 13(2), 276–84.
Walker, L. C., Price, D. L., Voytko, M. L., and Schenk, D. B. (1994) Labelling of cerebral amyloid in vivo with a monoclonal antibody. J. Neuropathol. Exp. Neurol. 53(4), 377–83.
Poduslo, J. F., Ramakrishnan, M., Holasek, S. S., Ramirez-Alvarado, M., Kandimalla, K. K., Gilles, E. J., Curran, G. L., and Wengenack, T. M. (2007) In vivo targeting of antibody fragments to the nervous system for Alzheimer’s disease immunotherapy and molecular imaging of amyloid plaques. J. Neurochem. 102(2), 420–33.
Shi, J., Perry, G., Berridge, M. S., Aliev, G., Siedlak, S. L., Smith, M. A., LaManna, J. C., and Friedland, R. P. (2002) Labeling of cerebral amyloid beta deposits in vivo using intranasal basic fibroblast growth factor and serum amyloid P component in mice. J. Nucl. Med. 43(8), 1044–51.
Klunk, W. E., Wang, Y., Huang, G. F., Debnath, M. L., Holt, D. P., and Mathis, C. A. (2001) Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 69(13), 1471–84.
Mathis, C. A., Klunk, W. E., Price, J. C., and DeKosky, S. T. (2005) Imaging technology for neurodegenerative diseases: progress toward detection of specific pathologies. Arch Neurol. 62(2), 196–200.
Ye, L., Morgenstern, J. L., Gee, A. D., Hong, G., Brown, J., and Lockhart, A. (2005) Delineation of positron emission tomography imaging agent binding sites on beta-amyloid peptide fibrils. J. Biol. Chem. 280(25), 23599–604.
Lockhart, A., Ye, L., Judd, D. B., Merritt, A. T., Lowe, P. N., Morgenstern, J. L., Hong, G., Gee, A. D., and Brown, J. (2005) Evidence for the presence of three distinct binding sites for the thioflavin T class of Alzheimer’s disease PET imaging agents on beta-amyloid peptide fibrils. J. Biol. Chem. 280(9), 7677–84.
Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D. P., Bergstrom, M., Savitcheva, I., Huang, G. F., Estrada, S., et al. (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55(3), 306–19.
Price, J. C., Klunk, W. E., Lopresti, B. J., Lu, X., Hoge, J. A., Ziolko, S. K., Holt, D. P., Meltzer, C. C., DeKosky, S. T., and Mathis, C. A. (2005) Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab. 25(11), 1528–47.
Rowe, C. C., Ng, S., Ackermann, U., Gong, S. J., Pike, K., Savage, G., Cowie, T. F., Dickinson, K. L., Maruff, P., Darby, D., et al. (2007) Imaging beta-amyloid burden in aging and dementia. Neurology 68(20), 1718–25.
Verhoeff, N. P., Wilson, A. A., Takeshita, S., Trop, L., Hussey, D., Singh, K., Kung, H. F., Kung, M. P., and Houle, S. (2004) In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am. J. Geriatr. Psychiatry 12(6), 584–95.
Price, J. C., Klunk, W. E., Lopresti, B. J., Lu, X., Hoge, J. A., Ziolko, S. K., Holt, D. P., Meltzer, C. C., Dekosky, S. T., and Mathis, C. A. (2005) Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab. 25, 1528–47.
Buckner, R. L., Snyder, A. Z., Shannon, B. J., LaRossa, G., Sachs, R., Fotenos, A. F., Sheline, Y. I., Klunk, W. E., Mathis, C. A., Morris, J. C., et al. (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25(34), 7709–17.
Villemagne, V. L., Fodero-Tavoletti, M. T., Pike, K. E., Cappai, R., Masters, C. L., and Rowe, C. C. (2008) The ART of loss: Abeta imaging in the evaluation of Alzheimer’s disease and other dementias. Mol. Neurobiol. DOI 10.1007/s12035-008-8019-y
Archer, H. A., Edison, P., Brooks, D. J., Barnes, J., Frost, C., Yeatman, T., Fox, N. C., and Rossor, M. N. (2006) Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann. Neurol. 60(1), 145–7.
Fagan, A. M., Mintun, M. A., Mach, R. H., Lee, S. Y., Dence, C. S., Shah, A. R., Larossa, G. N., Spinner, M. L., Klunk, W. E., Mathis, C. A., et al. (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta(42) in humans. Ann. Neurol. 59(3), 512–9.
Mintun, M. A., Larossa, G. N., Sheline, Y. I., Dence, C. S., Lee, S. Y., Mach, R. H., Klunk, W. E., Mathis, C. A., DeKosky, S. T., and Morris, J. C. (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67(3), 446–52.
Price, J. L. and Morris, J. C. (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 45(3), 358–68.
Hulette, C. M., Welsh-Bohmer, K. A., Murray, M. G., Saunders, A. M., Mash, D. C., and McIntyre, L. M. (1998) Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J. Neuropathol. Exp. Neurol. 57(12), 1168–74.
Morris, J. C. and Price, A. L. (2001) Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J. Mol. Neurosci. 17(2), 101–18.
Rabinovici, G. D., Furst, A. J., O’Neil, J. P., Racine, C. A., Mormino, E. C., Baker, S. L., Chetty, S., Patel, P., Pagliaro, T. A., Klunk, W. E., et al. (2007) 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 68(15), 1205–12.
Engler, H., Santillo, A. F., Wang, S. X., Lindau, M., Savitcheva, I., Nordberg, A., Lannfelt, L., Langstrom, B., and Kilander, L. (2007) In vivo amyloid imaging with PET in frontotemporal dementia. Eur. J. Nucl. Med. Mol. Imaging 35(1), 100–6.
Drzezga, A., Grimmer, T., Henriksen, G., Stangier, I., Perneczky, R., Diehl-Schmid, J., Mathis, C. A., Klunk, W. E., Price, J., DeKosky, S., et al. (2008) Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage 39(2), 619–33.
Yaffe, K., Petersen, R. C., Lindquist, K., Kramer, J., and Miller, B. (2006) Subtype of mild cognitive impairment and progression to dementia and death. Dement. Geriatr. Cogn. Disord. 22(4), 312–9.
Pike, K. E., Savage, G., Villemagne, V. L., Ng, S., Moss, S. A., Maruff, P., Mathis, C. A., Klunk, W. E., Masters, C. L., and Rowe, C. C. (2007) {beta}-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130(11), 2837–44.
Villemagne, V. L., Pike, K. E., Darby, D., Maruff, P., Savage, G., Ng, S., Ackermann, U., Cowie, T. F., Currie, J., Chan, S. G., et al. (2008) Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia 46(6), 1688–97.
Ng, S., Villemagne, V. L., Berlangieri, S., Lee, S. T., Cherk, M., Gong, S. J., Ackermann, U., Saunder, T., Tochon-Danguy, H., Jones, G., et al. (2007) Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J. Nucl. Med. 48(4), 547–52.
Rowe, C. C., Ackerman, U., Browne, W., Mulligan, R., Pike, K. L., O’Keefe, G., Tochon-Danguy, H., Chan, G., Berlangieri, S. U., Jones, G., et al. (2008) Imaging of amyloid beta in Alzheimer’s disease with (18)F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 7(2), 129–35.
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. (1991) Comparing functional (PET) images: The assessment of significant change. J. Cereb. Blood Flow Metab. 11, 690–9.
Minoshima, S., Koeppe, R. A., Frey, K. A., Ishihara, M., and Kuhl, D. E. (1994) Stereotactic PET atlas of the human brain: aid for visual interpretation of functional brain images. J. Nucl. Med. 35(6), 949–54.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., and Joliot, M. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–89.
McKeith, I. G., Galasko, D., Kosaka, K., Perry, E. K., Dickson, D. W., Hansen, L. A., Salmon, D. P., Lowe, J., Mirra, S. S., Byrne, E. J., et al. (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47(5), 1113–24.
McKhann, G. M., Albert, M. S., Grossman, M., Miller, B., Dickson, D., and Trojanowski, J. Q. (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 58(11), 1803–9.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 56, 303–8.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12(3), 189–98.
Morris, J. C. (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(11), 2412–4.
Wilson, A. A., Garcia, A., Chestakova, A., Kung, H., and Houle, S. (2004) A rapid one-step radiosynthesis of the β-amyloid imaging radiotracer N-methyl-[11C]2(40-methylaminophenyl)-6-hydroxybenzothiazole ([11C]-6-OH-BTA-1). J. Label. Compd. Radiopharm. 47, 679–82.
Lopresti, B. J., Klunk, W. E., Mathis, C. A., Hoge, J. A., Ziolko, S. K., Lu, X., Meltzer, C. C., Schimmel, K., Tsopelas, N. D., DeKosky, S. T., et al. (2005) Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J. Nucl. Med. 46(12), 1959–72.
Daube-Witherspoon, M. W., Matej, S., Karp, J. S., and Lewitt, R. M. (2001) Application of the 3D row action maximum likelihood algorithm to clinical PET imaging. IEEE Trans. Nucl. Sci. 48(1), 24–30.
Logan, J., Fowler, J. S., Volkow, N. D., Wang, G. J., Ding, Y. S., and Alexoff, D. L. (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 16, 834–40.
Mintun, M. A., Raichle, M. E., Kilbourn, M. R., Wooten, G. F., and Welch, M. J. (1984) A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann. Neurol. 15(3), 217–27.
Joachim, C. L., Morris, J. H., and Selkoe, D. J. (1989) Diffuse senile plaques occur commonly in the cerebellum in Alzheimer’s disease. Am. J. Pathol. 135(2), 309–19.
Mazziotta, J. C., Toga, A. W., Evans, A., Fox, P., and Lancaster, J. (1995) A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Map** (ICBM). Neuroimage 2(2), 89–101.
Carson, R. E. (1996) Mathematical modeling and compartmental analysis. In Nuclear medicine, diagnosis and therapy, Harbert, J., Eckelman, W. C., and Neumann, R., (Eds.). Thieme Medical, New York, NY, pp. 167–94.
Logan, J., Fowler, J. S., Volkow, N. D., Wolf, A. P., Dewey, S. L., Schyler, D. J., Macgregor, R. R., Hitzemann, R., Bendriem, B., and Gatley, S. J. (1990) Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 10, 740–7.
Yaqub, M., Tolboom, N., Boellaard, R., van Berckel, B. N., van Tilburg, E. W., Luurtsema, G., Scheltens, P., and Lammertsma, A. A. (2008) Simplified parametric methods for [(11)C]PIB studies. Neuroimage 42(1), 76–86.
Mueller, S. G., Weiner, M. W., Thal, L. J., Petersen, R. C., Jack, C. R., Jagust, W., Trojanowski, J. Q., Toga, A. W., and Beckett, L. (2005) Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 1(1), 55–66.
Yee, S., Mathis, C., Klunk, W., Weissfeld, L., Lopresti, B., Bi, W., Ziolko, S., Berginc, M., DeKosky, S., and Price, J. (2007) Optimal time window for standardized uptake ratio as a simplified measure of PIB retension. J. Nucl. Med. 48, 404p.
McNamee, R. L., Yee, S. H., Price, J. C., Klunk, W. E., Rosario, B., Weissfeld, L., Ziolko, S., Berginc, M., Lopresti, B., Dekosky, S., et al. (2009) Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J. Nucl. Med. 50(3), 348–55.
Koivunen, J., Verkkoniemi, A., Aalto, S., Paetau, A., Ahonen, J. P., Viitanen, M., Nagren, K., Rokka, J., Haaparanta, M., Kalimo, H., et al. (2008) PET amyloid ligand [11C]PIB uptake shows predominantly striatal increase in variant Alzheimer’s disease. Brain 131(Pt 7), 1845–53.
Acknowledgments
Supported in part by funds from the NHMRC Grant #509166, Austin Hospital Medical Research Foundation, Neurosciences Victoria, and the University of Melbourne.
We thank Dr. Henri Tochon-Danguy, Dr. Gordon Chan, Dr. Uwe Ackermann, Dr. Kerryn Pike, Dr. Kenneth Young, Dr. Sylvia Gong, Mr. Tim Saunder, Mr. Gareth Jones, Mrs. Jessica Sagona, Mrs. Kunthi Pathmaraj, Ms. Bridget Chappell, and Mr. Jason Bradley for their crucial role in our ongoing research projects.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Humana Press
About this protocol
Cite this protocol
Villemagne, V.L., O’Keefe, G., Mulligan, R.S., Rowe, C.C. (2011). Quantitative Approaches to Amyloid Imaging. In: Shah, K. (eds) Molecular Imaging. Methods in Molecular Biology, vol 680. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-901-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-60761-901-7_14
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-900-0
Online ISBN: 978-1-60761-901-7
eBook Packages: Springer Protocols