Abstract
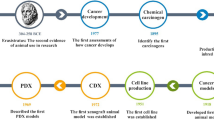
In spite of the latest advancements in understanding cancer development and progression, drugs successful in preclinical testing often fail upon reaching phase III clinical trials. A reason for this is the use of inappropriate preclinical models that do not preserve tumor heterogeneity. Although used for decades, cell cultures derived from patients substantially deviate from their original biopsy upon culturing; moreover, they cannot predict the response of an organism as a whole.
Patient-derived xenograft (PDX) models are emerging as powerful tools since they have a predictive therapeutic value and preserve the heterogeneity of the original tumors. PDX are established by implanting freshly isolated tumors from patients into immunocompromised mice, allowing for the progressive growth and amplification of cancer tissue for in vivo testing. Here, we describe the detailed methods we developed to establish PDX from both surgically removed endometrial cancer fragments (endometrial cancer) and fine-needle aspiration biopsies (pancreatic cancer).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Byrne AT, Alférez DG, Amant F et al (2017) Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 17:254–268
Bertotti A, Migliardi G, Galimi F et al (2011) A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1:508–523
Depreeuw J, Hermans E, Schrauwen S et al (2015) Characterization of patient-derived tumor xenograft models of endometrial cancer for preclinical evaluation of targeted therapies. Gynecol Oncol 139:118–126
Bruna A, Rueda OM, Greenwood W et al (2016) A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell 167:260–274.e22
DeRose YS, Wang G, Lin YC et al (2011) Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17:1514–1520
Julien S, Merino-Trigo A, Lacroix L et al (2012) Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res 18:5314–5328
Rubio-Viqueira B, Jimeno A, Cusatis G et al (2006) An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 12:4652–4661
Leucci E, Vendramin R, Spinazzi M et al (2016) Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531:518–522. https://doi.org/10.1038/nature17161
Hermans E, Van der Merwe SW, Depreeuw J et al (2016) Successful application of endoscopic ultrasound-guided fine needle biopsy to establish pancreatic patient-derived tumor xenografts: a pilot study. Endoscopy 48(11):1016–1022
Acknowledgment
Trace, the KU Leuven PDX Platform has been established thanks to grants from the National Kanker Plan (NKP), Belgium, and the Verelst Uterine Cancer Fund Leuven (VBL). Trace also acknowledges the support of the Stichting tegen Kanker Foundation and is part of the EuroPDX Consortium. FA is a senior researcher for the Research Fund Flanders (F.W.O.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Annibali, D., Leucci, E., Hermans, E., Amant, F. (2019). Development of Patient-Derived Tumor Xenograft Models. In: Fendt, SM., Lunt, S. (eds) Metabolic Signaling. Methods in Molecular Biology, vol 1862. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-8769-6_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-8769-6_15
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-8768-9
Online ISBN: 978-1-4939-8769-6
eBook Packages: Springer Protocols