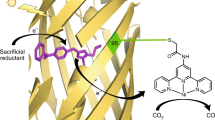
Abstract
Artificial photoenzymes with noncanonical photo-redox cofactors have paved the way for enzyme rational design and the creation of new-to-nature biocatalysts. Genetically encoded photo-redox cofactors endow photoenzymes with enhanced or novel activities that catalyze numerous transformations with high efficiency. Herein, we describe a protocol of repurposing photosensitizer proteins (PSP) through genetic code expansion to facilitate multiple photocatalytic conversions including photo-activated dehalogenation of aryl halides, CO2 to CO and CO2 to formic acid reduction. The methods for expression, purification, and characterization of the PSP are detailed. The installation of the catalytic modules and the utilization of PSP-based artificial photoenzymes for photoenzymatic CO2 reduction and dehalogenation are also described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Tibrewal N, Tang Y (2014) Biocatalysts for natural product biosynthesis. Annu Rev Chem Biomol Eng 5:347–366. https://doi.org/10.1146/annurev-chembioeng-060713-040008
Devine PN, Howard RM, Kumar R, Thompson MP, Truppo MD, Turner NJ (2018) Extending the application of biocatalysis to meet the challenges of drug development. Nat Rev Chem 2(12):409–421. https://doi.org/10.1038/s41570-018-0055-1
Taylor A, Heyes DJ, Scrutton NS (2022) Catalysis by Nature’s photoenzymes. Curr Opin Struct Biol 77:102491. https://doi.org/10.1016/j.sbi.2022.102491
Heyes DJ, Zhang S, Taylor A, Johannissen LO, Hardman SJO, Hay S, Scrutton NS (2021) Photocatalysis as the ‘master switch’ of photomorphogenesis in early plant development. Nat Plants 7(3):268–276. https://doi.org/10.1038/s41477-021-00866-5
Sancar A (2003) Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103(6):2203–2237. https://doi.org/10.1021/cr0204348
Sorigue D, Legeret B, Cuine S, Blangy S, Moulin S, Billon E, Richaud P, Brugiere S, Coute Y, Nurizzo D, Muller P, Brettel K, Pignol D, Arnoux P, Li-Beisson Y, Peltier G, Beisson F (2017) An algal photoenzyme converts fatty acids to hydrocarbons. Science 357(6354):903–907. https://doi.org/10.1126/science.aan6349
Sorigue D, Hadjidemetriou K, Blangy S, Gotthard G, Bonvalet A, Coquelle N, Samire P, Aleksandrov A, Antonucci L, Benachir A, Boutet S, Byrdin M, Cammarata M, Carbajo S, Cuine S, Doak RB, Foucar L, Gorel A, Grunbein M, Hartmann E, Hienerwadel R, Hilpert M, Kloos M, Lane TJ, Legeret B, Legrand P, Li-Beisson Y, Moulin SLY, Nurizzo D, Peltier G, Schiro G, Shoeman RL, Sliwa M, Solinas X, Zhuang B, Barends TRM, Colletier JP, Joffre M, Royant A, Berthomieu C, Weik M, Domratcheva T, Brettel K, Vos MH, Schlichting I, Arnoux P, Muller P, Beisson F (2021) Mechanism and dynamics of fatty acid photodecarboxylase. Science 372(6538):148. https://doi.org/10.1126/science.abd5687
Miller DC, Athavale SV, Arnold FH (2022) Combining chemistry and protein engineering for new-to-nature biocatalysis. Nat Synth 1(1):18–23. https://doi.org/10.1038/s44160-021-00008-x
Lovelock SL, Crawshaw R, Basler S, Levy C, Baker D, Hilvert D, Green AP (2022) The road to fully programmable protein catalysis. Nature 606(7912):49–58. https://doi.org/10.1038/s41586-022-04456-z
Lee SH, Choi DS, Kuk SK, Park CB (2018) Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons. Angew Chem Int Ed Engl 57(27):7958–7985. https://doi.org/10.1002/anie.201710070
Hutton GA, Reuillard B, Martindale BC, Caputo CA, Lockwood CW, Butt JN, Reisner E (2016) Carbon dots as versatile photosensitizers for solar-driven catalysis with redox enzymes. J Am Chem Soc 138(51):16722–16730. https://doi.org/10.1021/jacs.6b10146
Kim J, Lee SH, Tieves F, Choi DS, Hollmann F, Paul CE, Park CB (2018) Biocatalytic C=C bond reduction through carbon nanodot-sensitized regeneration of NADH analogues. Angew Chem Int Ed Engl 57(42):13825–13828. https://doi.org/10.1002/anie.201804409
van Schie MMCH, Zhang W, Tieves F, Choi DS, Park CB, Burek BO, Bloh JZ, Arends IWCE, Paul CE, Alcalde M, Hollmann F (2019) Cascading g-C3N4 and peroxygenases for selective oxyfunctionalization reactions. ACS Catal 9(8):7409–7417. https://doi.org/10.1021/acscatal.9b01341
Zhang SH, Shi JF, Sun YY, Wu YZ, Zhang YS, Cai ZY, Chen YX, You C, Han PP, Jiang ZY (2019) Artificial thylakoid for the coordinated photoenzymatic reduction of carbon dioxide. ACS Catal 9(5):3913–3925. https://doi.org/10.1021/acscatal.9b00255
Zhang SH, Shi JF, Chen YX, Huo Q, Li WR, Wu YZ, Sun YY, Zhang YS, Wang XD, Jiang ZY (2020) Unraveling and manipulating of NADH oxidation by photogenerated holes. ACS Catal 10(9):4967–4972. https://doi.org/10.1021/acscatal.0c00471
Yadav RK, Baeg JO, Oh GH, Park NJ, Kong KJ, Kim J, Hwang DW, Biswas SK (2012) A photocatalyst-enzyme coupled artificial photosynthesis system for solar energy in production of formic acid from CO2. J Am Chem Soc 134(28):11455–11461. https://doi.org/10.1021/ja3009902
Yoon J, Lee SH, Tieves F, Rauch M, Hollmann F, Park CB (2019) Light-harvesting dye-alginate hydrogel for solar-driven, sustainable biocatalysis of asymmetric hydrogenation. ACS Sustain Chem Eng 7(6):5632–5637. https://doi.org/10.1021/acssuschemeng.9b01075
Cesana PT, Li BX, Shepard SG, Ting SI, Hart SM, Olson CM, Alvarado JIM, Son MJ, Steiman TJ, Castellano FN, Doyle AG, MacMillan DWC, Schlau-Cohen GS (2022) A biohybrid strategy for enabling photoredox catalysis with low-energy light. Chem 8(1):174–185. https://doi.org/10.1016/j.chempr.2021.10.010
Wang L, **e J, Schultz PG (2006) Expanding the genetic code. Annu Rev Biophys Biomol Struct 35:225–249. https://doi.org/10.1146/annurev.biophys.35.101105.121507
**e J, Schultz PG (2006) A chemical toolkit for proteins – an expanded genetic code. Nat Rev Mol Cell Biol 7(10):775–782. https://doi.org/10.1038/nrm2005
Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79:413–444. https://doi.org/10.1146/annurev.biochem.052308.105824
Hu C, Wang J (2016) Method for enzyme design with genetically encoded unnatural amino acids. In: Pecoraro VL (ed) Peptide, protein and enzyme design. Methods in enzymology, vol 580, pp 109–133. doi:https://doi.org/10.1016/bs.mie.2016.06.005
Yu Y, Liu X, Wang J (2019) Expansion of redox chemistry in designer metalloenzymes. Acc Chem Res 52(3):557–565. https://doi.org/10.1021/acs.accounts.8b00627
Pagar AD, Patil MD, Flood DT, Yoo TH, Dawson PE, Yun H (2021) Recent advances in biocatalysis with chemical modification and expanded amino acid alphabet. Chem Rev 121(10):6173–6245. https://doi.org/10.1021/acs.chemrev.0c01201
Dorman G, Nakamura H, Pulsipher A, Prestwich GD (2016) The life of pi star: exploring the exciting and forbidden worlds of the benzophenone Photophore. Chem Rev 116(24):15284–15398. https://doi.org/10.1021/acs.chemrev.6b00342
Chin JW, Martin AB, King DS, Wang L, Schultz PG (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. Proc Natl Acad Sci U S A 99(17):11020–11024. https://doi.org/10.1073/pnas.172226299
Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang Z, Schultz PG (2003) An expanded eukaryotic genetic code. Science 301(5635):964–967. https://doi.org/10.1126/science.1084772
Hino N, Okazaki Y, Kobayashi T, Hayashi A, Sakamoto K, Yokoyama S (2005) Protein photo-cross-linking in mammalian cells by site-specific incorporation of a photoreactive amino acid. Nat Methods 2(3):201–206. https://doi.org/10.1038/nmeth739
Coin I, Katritch V, Sun T, **ang Z, Siu FY, Beyermann M, Stevens RC, Wang L (2013) Genetically encoded chemical probes in cells reveal the binding path of urocortin-I to CRF class B GPCR. Cell 155(6):1258–1269. https://doi.org/10.1016/j.cell.2013.11.008
Chen L, Zhu C, Guo H, Li R, Zhang L, **ng Z, Song Y, Zhang Z, Wang F, Liu X, Zhang Y, Ma RZ, Wang F (2020) Epitope-directed antibody selection by site-specific photocrosslinking. Sci Adv 6(14):eaaz7825. https://doi.org/10.1126/sciadv.aaz7825
Mori H, Ito K (2006) Different modes of SecY-SecA interactions revealed by site-directed in vivo photo-cross-linking. Proc Natl Acad Sci U S A 103(44):16159–16164. https://doi.org/10.1073/pnas.0606390103
Bruninghoff K, Aust A, Taupitz KF, Wulff S, Dorner W, Mootz HD (2020) Identification of SUMO binding proteins enriched after covalent photo-cross-linking. ACS Chem Biol 15(9):2406–2414. https://doi.org/10.1021/acschembio.0c00609
Sun N, Huang J, Qian J, Zhou TP, Guo J, Tang L, Zhang W, Deng Y, Zhao W, Wu G, Liao RZ, Chen X, Zhong F, Wu Y (2022) Enantioselective [2+2]-cycloadditions with triplet photoenzymes. Nature 611(7937):715–720. https://doi.org/10.1038/s41586-022-05342-4
Trimble JS, Crawshaw R, Hardy FJ, Levy CW, Brown MJB, Fuerst DE, Heyes DJ, Obexer R, Green AP (2022) A designed photoenzyme for enantioselective [2+2] cycloadditions. Nature 611(7937):709–714. https://doi.org/10.1038/s41586-022-05335-3
Fu Y, Huang J, Wu Y, Liu X, Zhong F, Wang J (2021) Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase. J Am Chem Soc 143(2):617–622. https://doi.org/10.1021/jacs.0c10882
Liu X, Kang F, Hu C, Wang L, Xu Z, Zheng D, Gong W, Lu Y, Ma Y, Wang J (2018) A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO(2)-reducing enzyme. Nat Chem 10(12):1201–1206. https://doi.org/10.1038/s41557-018-0150-4
Kang F, Yu L, **a Y, Yu M, **a L, Wang Y, Yang L, Wang T, Gong W, Tian C, Liu X, Wang J (2021) Rational design of a miniature photocatalytic CO2-reducing enzyme. ACS Catal 11(9):5628–5635. https://doi.org/10.1021/acscatal.1c00287
Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67:509–544. https://doi.org/10.1146/annurev.biochem.67.1.509
Yang F, Moss LG, Phillips GN Jr (1996) The molecular structure of green fluorescent protein. Nat Biotechnol 14(10):1246–1251. https://doi.org/10.1038/nbt1096-1246
Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS (2006) Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24(1):79–88. https://doi.org/10.1038/nbt1172
Craggs TD (2009) Green fluorescent protein: structure, folding and chromophore maturation. Chem Soc Rev 38(10):2865–2875. https://doi.org/10.1039/b903641p
Kuehnel MF, Orchard KL, Dalle KE, Reisner E (2017) Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J Am Chem Soc 139(21):7217–7223. https://doi.org/10.1021/jacs.7b00369
Shields BJ, Doyle AG (2016) Direct C(sp(3))-H cross coupling enabled by catalytic generation of chlorine radicals. J Am Chem Soc 138(39):12719–12722. https://doi.org/10.1021/jacs.6b08397
Dauter Z, Wilson KS, Sieker LC, Meyer J, Moulis JM (1997) Atomic resolution (0.94 A) structure of clostridium acidurici ferredoxin. Detailed geometry of [4Fe-4S] clusters in a protein. Biochemistry 36(51):16065–16073. https://doi.org/10.1021/bi972155y
Acknowledgments
The authors are grateful to the National Key R&D Program of China (2021YFA0910802, 2019YFA0904002, 2019YFA0904103, 2020YFA0908503, 2020YFA0907701) and Sanming Project of Medicine in Shenzhen (no. SZSM201811092).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Wang, J., **a, Y., Guo, X. (2023). Repurposing Photosensitizer Proteins Through Genetic Code Expansion to Facilitate Photo-Biocatalysis. In: Tsai, YH., Elsässer, S.J. (eds) Genetically Incorporated Non-Canonical Amino Acids. Methods in Molecular Biology, vol 2676. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3251-2_3
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3251-2_3
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3250-5
Online ISBN: 978-1-0716-3251-2
eBook Packages: Springer Protocols