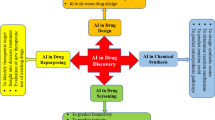
Abstract
The continuous development and progress of biotechnology and information technology provides data for pharmaceutical research and application. It is difficult to fully utilize large-scale data with simple statistical analysis methods. In order to improve data utilization, pharmaceutical research must be promoted using advanced information analysis. Artificial intelligence has experienced half a century of development since its inception and has been successfully applied to many industrial and technological fields. Recently, breakthroughs in machine learning represented by deep learning have made artificial intelligence one of the most popular research directions. Artificial intelligence algorithms use different types of data based on various strategies to do multiple tasks such as search and discrimination, and are suitable for solving massive data analysis problems faced in network pharmacological research. This chapter briefly introduces artificial intelligence algorithms and their applications in network pharmacology research, and provides references for researchers to better understand and apply artificial intelligence.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–90.
Zhang Y. Progress in network pharmacology and modern research of traditional Chinese medicine. Chin J Pharmacol Toxicol. 2015;29(06):883–92 (in Chinese).
Maulik U, Bandyopadhyay S. Genetic algorithm-based clustering technique. 2000;33.
Selim S, Alsultan K. A simulated annealing algorithm for the clustering problem. 1991;24.
Zou L. Artificial intelligence and its development and application. Inf Network Secur. 2012(02):11–3 (in Chinese).
Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. Commun ACM. 2017;60(6):84–90.
Lecun Y, Boser B, Denker J, et al. Handwritten digit recognition with a back-propagation network. 1997;2.
Mikolov T, Deoras A, Povey D, et al. Strategies for training large scale neural network language models. 2011.
Mohamed A, Dahl GE, Hinton G. Acoustic modeling using deep belief networks. IEEE Trans Audio Speech Lang Process. 2012;20(1):14–22.
Kalinin AA, Higgins GA, Reamaroon N, et al. Deep learning in pharmacogenomics: from gene regulation to patient stratification. Pharmacogenomics. 2018;19(7):629–50.
Gawehn E, Hiss JA, Schneider G. Deep learning in drug discovery. Mol Inf. 2016;35(1):3–14.
Kotsiantis S. Supervised machine learning: a review of classification techniques. 2007;31.
Lecun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44.
Jain AK, Murty MN, Flynn PJ. Data clustering: a review. ACM Comput Surv. 1999;31(3):264–323.
Van Der Maaten L, Postma E, Herik H. Dimensionality reduction: a comparative review. 2007;10.
Zhou W. Network construction technology in network pharmacology research. Int J Pharm Res. 2016;43(05):797–812 (in Chinese).
Akhmedov M, Kedaigle A, Chong RE, et al. PCSF: an R-package for network-based interpretation of high-throughput data. PLoS Comput Biol. 2017;13(7):e1005694.
Turing AM. Computing machinery and intelligence// computers and thought. American Association for Artificial Intelligence; 1950.
He H, Garcia EA. Learning from imbalanced data. 2009;21.
Altschul S, Gish W, Miller W, et al. Basic local alignment search tool. 1990;215.
O'Boyle NM, Banck M, James CA, et al. Open Babel: an open chemical toolbox. J Cheminformatics. 2011;3(1):33.
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009:455–61.
Case DA, Cheatham TE, Darden T, et al. The Amber biomolecular simulation programs. J Comput Chem. 2005;26(16):1668–88.
Iorio F, Tagliaferri R, Bernardo DD. Identifying network of drug mode of action by gene expression profiling. J Comput Biol. 2009;16(2):241–51.
Subramanian A, Narayan R, Corsello SM, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. 2017;171(6):1437–52.e17.
Van Der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–605.
Shekhar K, Lapan SW, Whitney IE, et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell. 2016;166(5):1308–23.e30.
Grimes M, Hall B, Foltz L, et al. Integration of protein phosphorylation, acetylation, and methylation data sets to outline lung cancer signaling networks. Sci Signal. 2018;11(531):eaaq1087.
Vidyasagar M. Identifying predictive features in drug response using machine learning: opportunities and challenges. Annu Rev Pharmacol Toxicol. 2015;55(1):15–34.
Gamazon E, Wheeler H, Shah K. A gene-based association method for map** traits using reference transcriptome data. 2015;47.
**ong J, Zhou T. Gene regulatory network inference from multifactorial perturbation data. 2012.
Yamanishi Y, Kotera M, Moriya Y, et al. DINIES: drug-target interaction network inference engine based on supervised analysis. Nucleic Acids Res. 2014;42(W1):W39–45.
Gopalan PK, Blei DM. Efficient discovery of overlap** communities in massive networks. Proc Natl Acad Sci. 2013;110(36):14534–9.
Chen H, Engkvist O, Wang Y, et al. The rise of deep learning in drug discovery. Drug Discov Today. 2018;23(6):1241–50.
Mayr A, Klambauer G, Unterthiner T, et al. Large-scale comparison of machine learning methods for drug target prediction on ChEMBL. Chem Sci. 2018;9(24):5441–51.
Korotcov A, Tkachenko V, Russo DP, et al. Comparison of deep learning with multiple machine learning methods and metrics using diverse drug discovery data sets. Mol Pharm. 2017;14(12):4462–75.
Teschendorff AE. Avoiding common pitfalls in machine learning omic data science. Nat Mater. 2019;18(5):422–7.
Bero SA, Muda AK, Choo YH, et al. Similarity measure for molecular structure: a brief review. J Phys: Conf Ser. 2017;892:012015.
Zhang Y, Cheng X, Zhou W. Drug reorientation: an important application field of network pharmacology. Chin J Pharmacol Toxicol. 2012;26(6):779–85 (in Chinese).
Willett P. The calculation of molecular structural similarity: principles and practice. Mol Inf. 2014;33(6–7):403–13.
Vilar S, Harpaz R, Uriarte E, et al. Drug—drug interaction through molecular structure similarity analysis. J Am Med Inform Assoc. 2012;19(6):1066–74.
Yan C, Wang J, Lan W, et al. SDTRLS: predicting drug-target interactions for complex diseases based on chemical substructures. Complexity. 2017;2017:1–10.
Keiser MJ, Setola V, Irwin JJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462(7270):175–81.
Neves BJ, Braga RC, Melo Filho CC, et al. QSAR-based virtual screening: advances and applications in drug discovery. Front Pharmacol. 2018;9:1275.
Chen R, Liu X, ** S, et al. Machine learning for drug-target interaction prediction. Molecules. 2018;23(9):2208.
Mitchell JBO. ChemInform abstract: the relationship between the sequence identities of helical proteins in the PDB and the molecular similarities of their ligands. ChemInform. 2002;33(10):no-no.
Bleakley K, Yamanishi Y. Supervised prediction of drug–target interactions using bipartite local models. Bioinformatics. 2009;25(18):2397–403.
Yamanishi Y, Araki M, Gutteridge A, et al. Prediction of drug–target interaction networks from the integration of chemical and genomic spaces. Bioinformatics. 2008;24(13):i232–40.
Michael J, et al. Predicting new molecular targets for known drugs. Nature. 2009;462(7270):175–81.
Martin YC, Kofron JL, Traphagen LM. Do structurally similar molecules have similar biological activity? J Med Chem. 2002;45(19):4350–8.
Schuffenhauer A, Floersheim P, Acklin P, et al. Similarity metrics for ligands reflecting the similarity of the target proteins. J Chem Inf Comput Sci. 2003;43(2):391–405.
Cheng F, et al. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput Biol. 2012;8(5):e1002503.
Zhao SW, Li S. Network-based relating pharmacological and genomic spaces for drug target identification. PLoS ONE. 2010;5:e11764.
Zhang L, Fourches D, Sedykh A, et al. Discovery of novel antimalarial compounds enabled by QSAR-based virtual screening. J Chem Inf Model. 2013;53(2):475–92.
Fan S, Li X. Reverse molecular docking: a new approach to discovery and identification of drug targets. Adv Physiol Sci. 2012;043(005):367–70 (in Chinese).
Kuhn M, Campillos M, González P, et al. Large-scale prediction of drug-target relationships. FEBS Lett. 2008;582(8):1283–90.
Hansen NT, Brunak S, Altman RB. Generating genome-scale candidate gene lists for pharmacogenomics. Clin Pharmacol Ther. 2009;86(2):183–9.
Kutalik Z, Beckmann JS, Bergmann S, et al. A modular approach for integrative analysis of large-scale gene-expression and drug-response data. Nat Biotechnol. 2008;26(5):531–9.
Chen YZ, Zhi DG. Ligand–protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins. 2001;43(2):217–26.
Li H, Gao Z, Kang L, et al. TarFisDock: a web server for identifying drug targets with docking approach. Nucleic Acids Res. 2006;34(Web Server):W219–W224.
Liu X, Ouyang S, Yu B, et al. PharmMapper server: a web server for potential drug target identification using pharmacophore map** approach. Nucleic Acids Res. 2010;38(Web Server):W609–W614.
Kinnings SL, Jackson RM. ReverseScreen3D: a structure-based ligand matching method to identify protein targets. J Chem Inf Model. 2011;51(3):624–34.
Wang JC, Chu PY, Chen CM, et al. idTarget: a web server for identifying protein targets of small chemical molecules with robust scoring functions and a divide-and-conquer docking approach. Nucleic Acids Res. 2012;40(W1):W393–9.
Yue QX, Cao ZW, Guan SH, et al. Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol Cell Proteomics. 2008;7(5):949–61.
Feng LX, **g CJ, Tang KL, et al. Clarifying the signal network of salvianolic acid B using proteomic assay and bioinformatic analysis†. PROTEOMICS. 2011;11(8):1473–85.
Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. J Urol. 2002;167(2, Part 2):1102–7.
Wu Z, Li W, Liu G, et al. Network-based methods for prediction of drug-target interactions. Front Pharmacol. 2018;9:1134.
Feixiong C, Chuang L, **g J, et al. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput Biol. 2012;8(5):e1002503.
Wu H, Miller E, Wijegunawardana D, et al. MD-Miner: a network-based approach for personalized drug repositioning. BMC Syst Biol. 2017;11(S5):86.
Isik Z, Baldow C, Cannistraci CV, et al. Drug target prioritization by perturbed gene expression and network information. Sci Rep. 2015;5(1):17417.
Melo Filho CC, Dantas RF, Braga RC, et al. QSAR-driven discovery of novel chemical scaffolds active against Schistosoma mansoni. J Chem Inf Model. 2016;56(7):1357–72.
Gomes MN, Braga RC, Grzelak EM, et al. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur J Med Chem. 2017;137:126–38.
Shen J, Tan C, Zhang Y, et al. Discovery of potent ligands for estrogen receptor β by structure-based virtual screening. J Med Chem. 2010;53(14):5361–5.
Hu G, Li X, Zhang X, et al. Discovery of inhibitors to block interactions of HIV-1 integrase with human LEDGF/p75 via structure-based virtual screening and bioassays. J Med Chem. 2012;55(22):10108–17.
Kumari P, Nath A, Chaube R. Identification of human drug targets using machine-learning algorithms. Comput Biol Med. 2015;56:175–81.
Zhang X, Li L, Ng MK, et al. Drug-target interaction prediction by integrating multiview network data. Comput Biol Chem. 2017;69:185–93.
Jamali AA, Ferdousi R, Razzaghi S, et al. DrugMiner: comparative analysis of machine learning algorithms for prediction of potential druggable proteins. Drug Discov Today. 2016;21(5):718–24.
Tang Y, Zhu W, Chen K, et al. New technologies in computer-aided drug design: toward target identification and new chemical entity discovery. Drug Discov Today: Technologies. 2006;3(3):307–13.
Rognan D. Structure-based approaches to target fishing and ligand profiling. Mol Inf. 2010;29(3):176–87.
Pireddu L, Poulin B, Szafron D, et al. Pathway analyst automated metabolic pathway prediction// IEEE Symposium on Computational Intelligence in Bioinformatics & Computational Biology. IEEE, 2005.
Dale JM, Popescu L, Karp PD. Machine learning methods for metabolic pathway prediction. BMC Bioinformatics. 2010;11(1):15.
Boudellioua I, Saidi R, Hoehndorf R, et al. Prediction of metabolic pathway involvement in prokaryotic UniProtKB data by association rule mining. PLoS ONE. 2016;11(7):e0158896.
Pang H, Lin A, Holford M, et al. Pathway analysis using random forests classification and regression. Bioinformatics. 2006;22(16):2028–36.
Hancock T, Mamitsuka H. A Markov classification model for metabolic pathways. Algorithms Mol Biol. 2010;5(1):10.
Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10(6):428–38.
Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10(7):507–19.
Campillos M, Kuhn M, Gavin AC, et al. Drug target identification using side-effect similarity. Science. 2008;321(5886):263–6.
Yang L, Agarwal P. Systematic drug repositioning based on clinical side-effects. P. Csermely. PLoS ONE. 2011;6(12):e28025.
Dimitri GM, Lió P. DrugClust: a machine learning approach for drugs side effects prediction. Comput Biol Chem. 2017;68:204–10.
Luo Y, Liu Q, Wu W, et al. Predicting drug side effects based on link prediction in bipartite network. Proceedings - 2014 7th International Conference on BioMedical Engineering and Informatics, BMEI 2014; 2015. p. 729–33.
Ferrero E, Agarwal P. Connecting genetics and gene expression data for target prioritisation and drug repositioning. BioData Mining. 2018;11(1):7.
Yin W, Gao C, Xu Y, et al. Learning opportunities for drug repositioning via GWAS and PheWAS findings. 2018.
Ye H, Liu Q, Wei J. Construction of drug network based on side effects and its application for drug repositioning. PLoS ONE. 2014;9(2):e87864.
Wang Y, Chen S, Deng N, et al. Drug repositioning by Kernel-based integration of molecular structure, molecular activity, and phenotype data. PLoS ONE. 2013;8(11):e78518.
Duan Q, Flynn C, Niepel M, et al. LINCS Canvas Browser: interactive web app to query, browse and interrogate LINCS L1000 gene expression signatures. Nucleic Acids Res. 2014;42(W1):W449–60.
Lamb J. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–35.
Keenan AB, Jenkins SL, Jagodnik KM, et al. The library of integrated network-based cellular signatures NIH program: system-level cataloging of human cells response to perturbations. Cell Syst. 2018;6(1):13–24.
Musa A, Ghoraie LS, Zhang SD, et al. A review of connectivity map and computational approaches in pharmacogenomics. Brief Bioinformatics. 2017:bbw112.
Iorio F, Rittman T, Ge H, et al. Transcriptional data: a new gateway to drug repositioning? Drug Discov Today. 2013;18(7–8):350–7.
**e L, He S, Wen Y, et al. Discovery of novel therapeutic properties of drugs from transcriptional responses based on multi-label classification. Sci Rep. 2017;7(1)
Young WC, Raftery AE, Yeung KY. A posterior probability approach for gene regulatory network inference in genetic perturbation data. Math Biosci Eng. 2016;13:1241–51.
Lee H, Kang S, Kim W. Drug repositioning for cancer therapy based on large-scale drug-induced transcriptional signatures. E. PLoS ONE. 2016;11(3):e0150460.
Sawada R, Iwata M, Tabei Y, et al. Predicting inhibitory and activatory drug targets by chemically and genetically perturbed transcriptome signatures. Sci Rep. 2018;8(1):156.
Edgar R, lash A. 6. The Gene Expression Omnibus (GEO): a gene expression and hybridization repository. 2002.
Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991.
Parkinson H, Kapushesky M, Shojatalab M, et al. ArrayExpress--a public database of microarray experiments and gene expression profiles. Nucleic Acids Res. 2007;35(Database issue):D747–50.
Luo H, Zhang P, Cao XH, et al. DPDR-CPI, a server that predicts drug positioning and drug repositioning via chemical-protein interactome. Sci Rep. 2016;6(1):35996.
Chen JJF, Visco DP. Develo** an in silico pipeline for faster drug candidate discovery: virtual high throughput screening with the signature molecular descriptor using support vector machine models. Chem Eng Sci. 2016:S0009250916300914.
Li H, Gao Z, Kang L, et al. TarFisDock: a web server for identifying drug targets with docking approach. Nucleic Acids Res. 2006;34(Web Server):W219–W224.
Luo H, Chen J, Shi L, et al. DRAR-CPI: a server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011;39(suppl_2):W492–W498.
Ruiz Carmona S, Alvarez-Garcia D, Foloppe N, et al. rDock: a fast, versatile and open source program for docking ligands to proteins and nucleic acids. PLoS Comput Biol. 2014;10:e1003571.
Lu L. Link prediction of complex networks. J UESTC. 2010;39(5):651–61 (in Chinese).
Chen X, Liu MX, Yan GY. Drug-target interaction prediction by random walk on the heterogeneous network. Mol Biosyst. 2012;8(7):1970.
Seal A, Ahn YY, Wild DJ. Optimizing drug-target interaction prediction based on random walk on heterogeneous networks. J Cheminform. 2015;7:40.
Zhang Y, Feng Y. Methods and development of link prediction. Measure Control Technol. 2019; 38(2):8–12 (in Chinese).
Liu W, Lü LY. Link prediction based on local random walk. EPL (Europhys Lett). 2010;89(5):58007.
Chen B. Link prediction of complex networks and its application in recommendation. Nan**g University of Aeronautics and Astronautics, 2016;126 (in Chinese).
Lorrain F, White HC. Structural equivalence of individuals in social networks. Soc Networks. 1977;1(1):67–98.
Chowdhury G. Introduction to modern information retrieval. McGraw Hill; 1983.
Zhang W, Huai Y, Miao Z, et al. Systems pharmacology for investigation of the mechanisms of action of traditional chinese medicine in drug discovery. Front Pharmacol. 2019;10
Wang J, Wu MY, Tan JQ, et al. High content screening for drug discovery from traditional Chinese medicine. Chin Med. 2019;14(1):5.
Xu HY, Zhang YQ, Liu ZM, et al. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019;47(Database issue):D976.
Liu BH, Gu YH, Tu Y, et al. Molecular regulative mechanisms of aging and interventional effects of Chinese herbal medicine. Zhongguo Zhong Yao Za Zhi. 2017;42(16):3065–71.
Chen X, Zhou H, Liu YB, et al. Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation. 2006.
Zhu Y, Gao B, Cui M. Design and implementation of TCM prescription analysis system framework. Chin J Tradit Chin Med. 2014;29(5):1543–46 (in Chinese).
Li S. conception and Research on biomarkers of TCM syndromes. J Tradit Chin Med. 2009(9):7–10 (in Chinese).
Li Q, Cheng T, Wang Y, et al. PubChem as a public resource for drug discovery. Drug Discov Today. 2010;15(23–24):1052–7.
Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–82.
Burley SK, Berman HM, Bhikadiya C, et al. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019;47(D1):D464–74.
Kuhn M, Letunic I, Jensen LJ, et al. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44(D1):D1075–9.
Edgar R, lash A. 6. The Gene Expression Omnibus (GEO): a gene expression and hybridization repository. 2002.
Tomczak K, Czerwińska P, Wiznerowicz M. Review the Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Współczesna Onkologia. 2015;1A:68–77.
Aliper A, Plis S, Artemov A, et al. Deep learning applications for predicting pharmacological properties of drugs and drug repurposing using transcriptomic data. Mol Pharm. 2016;13(7):2524–30.
Wang Z, Clark NR, Ma’ayan A. Drug-induced adverse events prediction with the LINCS L1000 data. Bioinformatics. 2016;32(15):2338–45.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhou, W., Li, X., Han, L., Fan, S. (2021). Application of Network Pharmacology Based on Artificial Intelligence Algorithms in Drug Development. In: Li, S. (eds) Network Pharmacology. Springer, Singapore. https://doi.org/10.1007/978-981-16-0753-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-0753-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0752-3
Online ISBN: 978-981-16-0753-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)