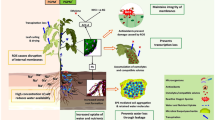
Abstract
Drought is accepted as one of the major constraints for crop growth, development and productivity worldwide. Drought-tolerant plants either employ drought avoidance mechanisms like elongation of root, reduction of leaf area and decreased stomatal number and conductance or drought tolerance mechanisms like accumulation of osmoprotectants (glycine betaine, proline, sugar alcohols, etc.) and other cellular and biochemical modifications. Another mechanism of drought tolerance in plants is their association with some beneficial rhizospheric or endophytic microbes. Plant growth-promoting (PGP) microbes help the crops to tolerate drought conditions by different mechanisms like secretion of phytohormones, exopolysaccharide and antioxidants, solubilization of essential micro and macro nutrients, accumulation of osmolytes and induction of stress-responsive genes. In this chapter, we review the role of PGP microbes in general stress mitigation strategies, with specific emphasis in imparting drought stress tolerance to plants.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abhilash P, Powell JR, Singh HB, Singh BK (2012) Plant–microbe interactions: novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol 30(8):416–420
Anjum SA, **e X-y, Wang L-c, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. African J Agric Res 6(9):2026–2032
Ansary MH, Rahmani HA, Ardakani MR, Paknejad F, Habibi D, Mafakheri S (2012) Effect of Pseudomonas fluorescent on proline and phytohormonal status of maize (Zea mays l.) under water deficit stress. Annals Biol Res 3(2):1054–1062
Arbona V, Manzi M, Ollas C, Gómez-Cadenas A (2013) Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci 14(3):4885–4911
Armada E, Roldán A, Azcon R (2014) Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb Ecol 67(2):410–420
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173(4):808–816
Athar H, Ashraf M (2009) Strategies for crop improvement against salinity and drought stress: an overview. In: Salinity and water stress. Springer, pp 1–16
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63(10):3523–3543
Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schäfer P, Schwarczinger I (2008) Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 180(2):501–510
Bano QU, Ilyas N, Bano A, Zafar NA, Akram AB, Hassan F (2013) Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak J Bot 45(S1):13–20
Bashan Y, de-Bashan LE, Prabhu S, Hernandez J-P (2014) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil 378(1–2):1–33
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI et al (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181(2):413–423
Bensalim S, Nowak J, Asiedu SK (1998) A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am J of Potato Res 75(3):145–152
Bhargava S, Sawant K (2013) Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed 132(1):21–32
Bian S, Jiang Y (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hortic 120(2):264–270
Biswas S, Kundu D, Mazumdar S, Saha A, Majumdar B, Ghorai A et al (2018) Study on the activity and diversity of bacteria in a new Gangetic alluvial soil (Eutrocrept) under rice-wheat-jute crop** system. J Environ Biol 39:379–386
Bouskill NJ, Wood TE, Baran R, Ye Z, Bowen BP, Lim H, Zhou J, Nostrand JDV, Nico P, Northen TR (2016) Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front Microbiol 7:525
Bresson J, Varoquaux F, Bontpart T, Touraine B, Vile D (2013) The PGPR strain Phyllobacterium brassicacearum STM 196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol 200(2):558–569
Caravaca F, Alguacil M, Hernández J, Roldán A (2005) Involvement of antioxidant enzyme and nitrate reductase activities during water stress and recovery of mycorrhizal myrtus communis and Phillyrea angustifolia plants. Plant Sci 169(1):191–197
Cassan F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45(1):12–19
Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5(3):250–257
Cho SM, Kang BR, Han SH, Anderson AJ, Park J-Y, Lee Y-H, Cho BH, Yang K-Y, Ryu C-M, Kim YC (2008) 2R, 3R-butanediol, a bacterial volatile produced by pseudomonas chlororaphis o6, is involved in induction of systemic tolerance to drought in arabidopsis thaliana. Mol Plant-Microbe Interact 21(8):1067–1075
Choudhary DK, Johri BN, Prakash A (2008) Volatiles as priming agents that initiate plant growth and defence responses. Curr Sci:595–604
Cohen AC, Bottini R, Pontin M, Berli FJ, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of aba levels. Physiol Plant 153(1):79–90
Cohen AC, Travaglia CN, Bottini R, Piccoli PN (2009) Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 87(5):455–462
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3(3):156–165
de Zelicourt A, Al-Yousif M, Hirt H (2013) Rhizosphere microbes as essential partners for plant stress tolerance. Mol Plant 6(2):242–245
Figueiredo MV, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40(1):182–188
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071
Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A (2014) Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol 201(3):916–927
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190(1):63–68
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5(4):355–377
Gou W, Tian L, Ruan Z, Zheng P, Chen F, Zhang L, Cui Z, Zheng P, Li Z, Gao M (2015) Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pak J Bot 47(2):581–586
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39(8):1968–1977
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom 2014
Hartmann M, Brunner I, Hagedorn F, Bardgett RD, Stierli B, Herzog C, Chen X, Zingg A, Graf-Pannatier E, Rigling A (2017) A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol Ecol 26(4):1190–1206
Hasanuzzaman M, Nahar K, Gill SS, Fujita M (2013) Drought stress responses in plants, oxidative stress, and antioxidant defense. In: Climate change and plant abiotic stress tolerance, pp 209–250
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Annal Microbiol 60(4):579–598
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agric Sci 11(1):57–61
Hueso S, García C, Hernández T (2012) Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biol Biochem 50:167–173
Hueso S, Hernández T, García C (2011) Resistance and resilience of the soil microbial biomass to severe drought in semiarid soils: the importance of organic amendments. Appl Soil Ecol 50:27–36
Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R (2009) Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agric Biol 11(1):100–105
Kasim WA, Osman ME, Omar MN, El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant-growth-promoting bacteria. Journal of Plant Growth Reg 32(1):122–130
Kaushal M, Wani SP (2016) Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Annal Microbiol 66(1):35–42
Khalvati M, Hu Y, Mozafar A, Schmidhalter U (2005) Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol 7(6):706–712
Khan M, Gemenet DC, Villordon A (2016) Root system architecture and abiotic stress tolerance: current knowledge in root and tuber crops. Front Plant Sci 7:1584
Kour D, Rana KL, Sheikh I, Kumar V, Yadav AN, Dhaliwal HS et al (2019a) Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc Natl Acad Sci India Sect B Biol Sci. https://doi.org/10.1007/s40011-019-01151-4
Kour D, Rana KL, Yadav AN, Yadav N, Kumar V, Kumar A et al (2019b) Drought-tolerant phosphorus-solubilizing microbes: biodiversity and biotechnological applications for alleviation of drought stress in plants. In: Sayyed RZ, Arora NK, Reddy MS (eds) Plant growth promoting Rhizobacteria for sustainable stress management: volume 1: Rhizobacteria in abiotic stress management. Springer, Singapore, pp 255–308. https://doi.org/10.1007/978-981-13-6536-2_13
Kour D, Rana KL, Yadav N, Yadav AN, Kumar A, Meena VS et al (2019c) Rhizospheric microbiomes: biodiversity, mechanisms of plant growth promotion, and biotechnological applications for sustainable agriculture. In: Kumar A, Meena VS (eds) Plant growth promoting Rhizobacteria for agricultural sustainability: from theory to practices. Springer, Singapore, pp 19–65. https://doi.org/10.1007/978-981-13-7553-8_2
Kour D, Rana KL, Yadav N, Yadav AN, Singh J, Rastegari AA et al (2019d) Agriculturally and industrially important fungi: current developments and potential biotechnological applications. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through Fungi, volume 2: perspective for value-added products and environments. Springer, Cham, pp 1–64. https://doi.org/10.1007/978-3-030-14846-1_1
Kumar M, Kour D, Yadav AN, Saxena R, Rai PK, Jyoti A et al (2019) Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 74:287–308. https://doi.org/10.2478/s11756-019-00190-6
Lata R, Chowdhury S, Gond SK, White JF Jr (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66(4):268–276
Lau JA, Lennon JT (2011) Evolutionary ecology of plant–microbe interactions: soil microbial structure alters selection on plant traits. New Phytol 192(1):215–224
Lim JH, Kim SD (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. The Plant Pathol J 29(2):201–208
Liu F, **ng S, Ma H, Du Z, Ma B (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97(20):9155–9164
Loreto F, Schnitzler J-P (2010) Abiotic stresses and induced bvocs. Trends Plant Sci 15(3):154–166
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annual Rev Microbiol 63:541–556
Marulanda A, Azcon R, Ruiz-Lozano JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119(4):526–533
Matilla MA, Ramos JL, Bakker PA, Doornbos R, Badri DV, Vivanco JM, Ramos-González MI (2010) Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation. Environ Microbiol Rep 2(3):381–388
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci:525–532
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166(2):525–530
Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh DP, Prabha R, Sahu PK, Gupta VK (2017) Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci 8:172
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467
Mintoo MN, Mishra S, Dantu PK (2019) Isolation and characterization of endophytic bacteria from Piper longum. Proc Natl Acad Sci India B Sect B. https://doi.org/10.1007/s40011-018-01064-8
Mishra S, Gupta D, Ranjan R (2019) Molecular approaches for enhancing abiotic stress tolerance in plants. In: Approaches for enhancing abiotic stress tolerance in plants. In: Hasanuzzaman M, Nahar K, Fujita M, Oku H, Islam T (eds) CRC Press, Taylor and Francis Group, Boca Raton. pp 387-404. https://doi.org/10.1201/9781351104722
Mitter B, Petric A, Shin MW, Chain PS, Hauberg-Lotte L, Reinhold-Hurek B, Nowak J, Sessitsch A (2013) Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front Plant Sci 4:120
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32(2):429–448
Naseem H, Bano A (2014) Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact 9(1):689–701
Naveed M, Hussain MB, Zahir ZA, Mitter B, Sessitsch A (2014a) Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul 73(2):121–131
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014b) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. Fd17. Environ Exp Bot 97:30–39
Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M (2007) The response of carbon metabolism and antioxidant defenses of Alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144(2):1104–1114
Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP (2014) Response of plants to water stress. Front Plant Sci 5:86
Pandey V, Ansari MW, Tula S, Yadav S, Sahoo RK, Shukla N, Bains G, Badal S, Chandra S, Gaur A (2016) Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 243(5):1251–1264
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441
Rahdari P, Hoseini S (2012) Drought stress: a review. Int J Agro Plant Prod 3(10):443–446
Rahdari P, Hosseini SM, Tavakoli S (2012) The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea l.) leaves. J Med Plants Res 6(9):1539–1547
Rodríguez-Salazar J, Suárez R, Caballero-Mellado J, Iturriaga G (2009) Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize Arabidopsis plants. FEMS Microbiol Lett 296(1):52–59
Rolli E, Marasco R, Vigani G, Ettoumi B, Mapelli F, Deangelis ML, Gandolfi C, Casati E, Previtali F, Gerbino R (2015) Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol 17(2):316–331
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in. Proc Natl Acad Sci 100(8):4927–4932
Salehi-Lisar SY, Bakhshayeshan-Agdam H (2016). Drought stress in plants: Causes, consequences, and tolerance. In: Drought stress tolerance in plants, vol 1, Springer, pp 1–16
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62(1):21–30
Sandhya V, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing pseudomonas putida strain gap-p45. Biol Fertil Soils 46(1):17–26
Schmidt R, Köberl M, Mostafa A, Ramadan EM, Monschein M, Jensen KB, Bauer R, Berg G (2014) Effects of bacterial inoculants on the indigenous microbiome and secondary metabolites of Chamomile plants. Front Microbiol 5:64
Selvakumar G, Panneerselvam P, Ganeshamurthy AN (2012) Bacterial mediated alleviation of abiotic stress in crops. In: Bacteria in agrobiology: stress management, Springer, pp 205–224
Sharma P, Khanna V, Kumari P (2013) Efficacy of aminocyclopropane-1-carboxylic acid (ACC)-deaminase-producing rhizobacteria in ameliorating water stress in chickpea under axenic conditions. Afr J of Microbiol Res 7(50):5749–5757
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58(2):221–227
Solano BR, Maicas JB, Mañero FG (2008) Physiological and molecular mechanisms of plant growth promoting rhizobacteria (PGPR). In: Plant-bacteria interactions: strategies and techniques to promote plant growth. Wiley, Weinheim, pp 41–52
Souza RD, Ambrosini A, Passaglia LM (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38(4):401–419
Suárez R, Wong A, Ramírez M, Barraza A, Orozco MC, Cevallos MA, Lara M, Hernández G, Iturriaga G (2008) Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol Plant-Microbe Interact 21(7):958–966
Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B (2010) Piriformospora indica confers drought tolerance in chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized cas protein. J Plant Physiol 167(12):1009–1017
Timmusk S, El-Daim IA, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Seisenbaeva G, Stenstrom E, Niinemets Ü (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9(5):e96086
Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6(1):1–14
Vargas L, Santa Brigida AB, Mota Filho JP, de Carvalho TG, Rojas CA, Vaneechoutte D, Van Bel M, Farrinelli L, Ferreira PC, Vandepoele K, Hemerly AS (2014) Drought tolerence conferred to sugarcane by association with Gluconacetobacter diazotrophicus: a transcriptomic view of hormone pathways. PLoS One 9(12)
Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2014) Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci 3:432–447
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56:44–58
Verma P, Yadav AN, Khannam KS, Mishra S, Kumar S, Saxena AK et al (2019) Appraisal of diversity and functional attributes of thermotolerant wheat associated bacteria from the peninsular zone of India. Saudi J Biol Sci 26:1882–1895. https://doi.org/10.1016/j.sjbs.2016.01.042
Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK et al (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899
Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK (2017) Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives: volume 2: microbial interactions and agro-ecological impacts. Springer, Singapore, pp 543–580. https://doi.org/10.1007/978-981-10-6593-4_22
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24
Wang CJ, Yang W, Wang C, Gu C, Niu DD, Liu HX, Wang YP, Guo JH (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One 7(12)
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52(suppl_1):487–511
Wu CH, Bernard SM, Andersen GL, Chen W (2009) Develo** microbe–plant interactions for applications in plant-growth promotion and disease control, production of useful compounds, remediation and carbon sequestration. Microbial Biotechnol 2(4):428–440
Xu P, Chen F, Mannas JP, Feldman T, Sumner LW, Roossinck MJ (2008) Virus infection improves drought tolerance. New Phytol 180(4):911–921
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-co2 response of stomata and its dependence on environmental factors. Front Plant Sci 7:657
Xu Z, Shimizu H, Ito S, Yagasaki Y, Zou C, Zhou G, Zheng Y (2014) Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239(2):421–435
Xu ZZ, Zhou GS (2006) Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass leymus chinensis. Planta 224(5):1080–1090
Yadav AN (2019) Microbiomes of wheat (Triticum aestivum L.) endowed with multifunctional plant growth promoting attributes. EC Microbiol 15:1–6
Yadav AN, Gulati S, Sharma D, Singh RN, Rajawat MVS, Kumar R et al (2019a) Seasonal variations in culturable archaea and their plant growth promoting attributes to predict their role in establishment of vegetation in Rann of Kutch. Biologia 74:1031–1043. https://doi.org/10.2478/s11756-019-00259-2
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B et al (2017a) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J Appl Biol Biotechnol 5:45–57
Yadav AN, Kumar V, Prasad R, Saxena AK, Dhaliwal HS (2018) Microbiome in Crops: Diversity, distribution and potential role in crops improvements. In: Prasad R, Gill SS, Tuteja N (eds) Crop improvement through microbial biotechnology. Elsevier, USA, pp 305–332
Yadav AN, Singh J, Rastegari AA, Yadav N (2020) Plant microbiomes for sustainable agriculture. Springer, Cham
Yadav AN, Singh S, Mishra S, Gupta A (2019b) Recent advancement in white biotechnology through fungi, Volume 2: perspective for value-added products and environments. Springer, Cham
Yadav AN, Singh S, Mishra S, Gupta A (2019c) Recent advancement in white biotechnology through fungi, Volume 3: perspective for sustainable environments. Springer, Cham
Yadav AN, Verma P, Kour D, Rana KL, Kumar V, Singh B et al (2017b) Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int J Environ Sci Nat Resour 3:1–8. https://doi.org/10.19080/IJESNR.2017.03.555601
Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A et al (2015) Diversity and phylogenetic profiling of niche-specific bacilli from extreme environments of India. Ann Microbiol 65:611–629
Yadav AN, Verma P, Singh B, Chauhan VS, Suman A, Saxena AK (2017c) Plant growth promoting Bacteria: biodiversity and multifunctional attributes for sustainable agriculture. Adv Biotechnol Microbiol 5:1–16
Yadav AN, Yadav N (2018) Stress-adaptive microbes for plant growth promotion and alleviation of drought stress in plants. Acta Sci Agric 2:85–88
Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14(1):1–4
Yang S, Vanderbeld B, Wan J, Huang Y (2010) Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol Plant 3(3):469–490
Zhang H, Kim M-S, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu C-M, Allen R, Melo IS (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226(4):839
Zhang H, Murzello C, Sun Y, Kim M-S, **e X, Jeter RM, Zak JC, Dowd SE, Paré PW (2010) Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis (GB03). Mol Plant-Microbe Interact 23(8):1097–1104
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jain, D., Phurailatpam, L., Mishra, S. (2020). Microbes-Mediated Mitigation of Drought Stress in Plants: Recent Trends and Future Challenges. In: Yadav, A., Rastegari, A., Yadav, N., Kour, D. (eds) Advances in Plant Microbiome and Sustainable Agriculture. Microorganisms for Sustainability, vol 20. Springer, Singapore. https://doi.org/10.1007/978-981-15-3204-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-3204-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3203-0
Online ISBN: 978-981-15-3204-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)