Abstract
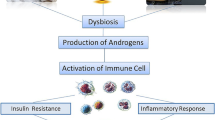
The gut microbiome contains trillions of commensal microorganisms that maintain a symbiotic relationship with the host, and its profound effects on gastrointestinal diseases have been widely described. Recently, gut microbiota have emerged as important factors in endocrine system diseases. Disruption of the gut microbiota affects neuroendocrine homeostasis and promotes peripheral endocrine system diseases, including obesity, diabetes, and hyperuricemia. This chapter provides a comprehensive overview of the biological mechanisms of gut microbiota that participate in endocrine system pathologies and discusses potential novel therapies for these diseases.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gentile CL, Weir TL (2018) The gut microbiota at the intersection of diet and human health. Science 362:776–780
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352
Kamada N, Seo SU, Chen GY, Nunez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321–335
Baumler AJ, Sperandio V (2016) Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93
Rook G, Backhed F, Levin BR, McFall-Ngai MJ, McLean AR (2017) Evolution, human-microbe interactions, and life history plasticity. Lancet 390:521–530
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230
Tremaroli V, Backhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249
Browne HP, Neville BA, Forster SC, Lawley TD (2017) Transmission of the gut microbiota: spreading of health. Nat Rev Microbiol 15:531–543
Postler TS, Ghosh S (2017) Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26:110–130
Lagier JC, Dubourg G, Million M, Cadoret F, Bilen M, Fenollar F, Levasseur A, Rolain JM, Fournier PE, Raoult D (2018) Culturing the human microbiota and culturomics. Nat Rev Microbiol 540–550
Mao K, Baptista AP, Tamoutounour S, Zhuang L, Bouladoux N, Martins AJ, Huang Y, Gerner MY, Belkaid Y, Germain RN (2018) Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554:255–259
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97
Newey PJ, Gorvin CM, Cleland SJ, Willberg CB, Bridge M, Azharuddin M, Drummond RS, van der Merwe PA, Klenerman P, Bountra C, Thakker RV (2013) Mutant prolactin receptor and familial hyperprolactinemia. N Engl J Med 369:2012–2020
Newell-Price J (2016) Pituitary gland: mortality in cushing disease. Nat Rev Endocr 12:502–503
Coon SL, Munson PJ, Cherukuri PF, Sugden D, Rath MF, Moller M, Clokie SJ, Fu C, Olanich ME, Rangel Z, Werner T, Program NCS, Mullikin JC, Klein DC (2012) Circadian changes in long noncoding RNAs in the pineal gland. Proc Natl Acad Sci USA 109:13319–13324
Hatori M, Hirota T, Iitsuka M, Kurabayashi N, Haraguchi S, Kokame K, Sato R, Nakai A, Miyata T, Tsutsui K, Fukada Y (2011) Light-dependent and circadian clock-regulated activation of sterol regulatory element-binding protein, X-box-binding protein 1, and heat shock factor pathways. Proc Natl Acad Sci USA 108:4864–4869
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin–a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93:350–384
De Leo S, Lee SY, Braverman LE (2016) Hyperthyroidism. Lancet 388:906–918
Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, Vidal-Puig A (2010) Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16:1001–1008
Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382
Vidal V, Sacco S, Rocha AS, da Silva F, Panzolini C, Dumontet T, Doan TM, Shan J, Rak-Raszewska A, Bird T, Vainio S, Martinez A, Schedl A (2016) The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev 30:1389–1394
Kohrle J, Jakob F, Contempre B, Dumont JE (2005) Selenium, the thyroid, and the endocrine system. Endocr Rev 26:944–984
Kim T, Loh YP (2005) Chromogranin A: a surprising link between granule biogenesis and hypertension. J Clin Investig 115:1711–1713
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171:372–384, e312
Trayhurn P (2013) Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93:1–21
Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W (2014) Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514:237–241
Khoshi A, Bajestani MK, Shakeri H, Goodarzi G, Azizi F (2019) Association of Omentin rs2274907 and FTO rs9939609 gene polymorphisms with insulin resistance in Iranian individuals with newly diagnosed type 2 diabetes. Lipids Health Dis 18:142
Gray SM, Niu J, Zhang A, Svendsen B, Campbell JE, D’Alessio DA, Tong J (2019) Intra-Islet ghrelin signaling does not regulate insulin secretion from adult mice. Diabetes 68(9):1795–1805
Zhou M, **g S, Wu CY, Shu L, Dong W, Liu Y, Chen M, Wynn RM, Wang J, Wang J, Gui WJ, Qi X, Lusis AJ, Li Z, Wang W, Ning G, Yang X, Chuang DT, Wang Y, Sun H (2019) Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68(9):1730–1746
Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B (2006) Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55:243–251
Chen PC, Inui A, Chen CY (2015) Paradoxical responses of plasma glucagon and bile acid levels after duodenal nutrient exclusion. Gut 64:516
Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P (2015) Autoimmune thyroid disorders. Autoimmun Rev 14:174–180
Nieman LK, Ilias I (2005) Evaluation and treatment of Cushing’s syndrome. Am J Med 118:1340–1346
Lodish M, Stratakis CA (2016) A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat Rev Endocr 12:255–262
Arregger AL, Cardoso EM, Zucchini A, Aguirre EC, Elbert A, Contreras LN (2014) Adrenocortical function in hypotensive patients with end stage renal disease. Steroids 84:57–63
de Loos WS (1996) Clinical problem-solving: identifying Addison’s disease. N Engl J Med 334:1403; author reply, 1404–1405
Matsumoto S, Hagiwara S, Kusaka J, Hasegawa R, Nonaka H, Shingu C, Noguchi T (2011) Catecholamine-resistant shock and hypoglycemic coma after cardiotomy in a patient with unexpected isolated ACTH deficiency. J Anesth 25:431–434
Savage DB, Petersen KF, Shulman GI (2007) Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87:507–520
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359:1151–1156
Yang Q, Vijayakumar A, Kahn BB (2018) Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol 19:654–672
Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R (2012) Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology 56:933–942
Johannsen DL, Tchoukalova Y, Tam CS, Covington JD, **e W, Schwarz J-M, Bajpeyi S, Ravussin E (2014) Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “Adipose Tissue Expandability” hypothesis. Diabetes Care 37:2789–2797
Buki KG, Bauer PI, Kun E (1997) Isolation and identification of a proteinase from calf thymus that cleaves poly(ADP-ribose) polymerase and histone H1. Biochem Biophys Acta 1338:100–106
So A, Thorens B (2010) Uric acid transport and disease. J Clin Investig 120:1791–1799
Carnovale C, Venegoni M, Clementi E (2014) Allopurinol overuse in asymptomatic hyperuricemia: a teachable moment. JAMA Intern Med 174:1031–1032
Jeon HJ, Oh J, Shin DH (2019) Urate-lowering agents for asymptomatic hyperuricemia in stage 3–4 chronic kidney disease: controversial role of kidney function. PLoS ONE 14:e0218510
El Aidy S, Dinan TG, Cryan JF (2015) Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther 37:954–967
Rieder R, Wisniewski PJ, Alderman BL, Campbell SC (2017) Microbes and mental health: a review. Brain Behav Immun 66:9–17
Martin-Villa JM (2014) Neuroendocrine stimulation of mucosal immune cells in inflammatory bowel disease. Curr Pharm Des 20:4766–4773
Caputi V, Giron MC (2018) Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci 19
Farzi A, Frohlich EE, Holzer P (2018) Gut microbiota and the neuroendocrine system. Neurotherapeutics 15:5–22
Roman P, Rueda-Ruzafa L, Cardona D, Cortes-Rodriguez A (2018) Gut-brain axis in the executive function of austism spectrum disorder. Behav Pharmacol 29:654–663
Hooks KB, Konsman JP, O’Malley MA (2018) Microbiota-gut-brain research: a critical analysis. Behav Brain Sci 1–40
Gerhardt S, Mohajeri MH (2018) Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 10
Moran CP, Shanahan F (2014) Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract Res Clin Gastroenterol 28:585–597
Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC (2015) Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37:984–995
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155
Kennedy PJ, Cryan JF, Dinan TG, Clarke G (2017) Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112:399–412
Bhattarai Y (2018) Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil 30:e13366
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345
Bercik P (2011) The microbiota-gut-brain axis: learning from intestinal bacteria? Gut 60:288–289
Nankova BB, Agarwal R, MacFabe DF, La Gamma EF (2014) Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells–possible relevance to autism spectrum disorders. PLoS ONE 9:e103740
Zhu H, Huang Q, Xu H, Niu L, Zhou JN (2009) Antidepressant-like effects of sodium butyrate in combination with estrogen in rat forced swimming test: involvement of 5-HT(1A) receptors. Behav Brain Res 196:200–206
Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977
Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW (2018) Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol Metab 11:70–83
Rastelli M, Cani PD, Knauf C (2019) The gut microbiome influences host endocrine functions. Endocr Rev 40(5):1271–1284
Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ (2018) Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun 70:48–60
Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A (2004) Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol 141:874–880
Das S, Sreevidya VS, Udvadia AJ, Gyaneshwar P (2019) Dopamine-induced sulfatase and its regulator are required for Salmonella enterica serovar Typhimurium pathogenesis. Microbiology 165:302–310
Strandwitz P (2018) Neurotransmitter modulation by the gut microbiota. Brain Res 1693:128–133
Cogan TA, Thomas AO, Rees LE, Taylor AH, Jepson MA, Williams PH, Ketley J, Humphrey TJ (2007) Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut 56:1060–1065
Hyland NP, Cryan JF (2010) A gut feeling about GABA: focus on GABA(B) receptors. Front Pharmacol 1:124
Mazzoli R, Pessione E (2016) The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol 7:1934
van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, El Aidy S (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10:310
Yunes RA, Poluektova EU, Dyachkova MS, Klimina KM, Kovtun AS, Averina OV, Orlova VS, Danilenko VN (2016) GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42:197–204
Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C (2012) gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113:411–417
Polovinkin L, Hassaine G, Perot J, Neumann E, Jensen AA, Lefebvre SN, Corringer PJ, Neyton J, Chipot C, Dehez F, Schoehn G, Nury H (2018) Conformational transitions of the serotonin 5-HT3 receptor. Nature 563:275–279
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276
Israelyan N, Del Colle A, Li Z, Park Y, **ng A, Jacobsen JPR, Luna RA, Jensen DD, Madra M, Saurman V, Rahim R, Latorre R, Law K, Carson W, Bunnett NW, Caron MG, Margolis KG (2019) Effects of serotonin and slow-release 5-HTP on gastrointestinal motility in a mouse model of depression. Gastroenterology 157(2)
Schneider S, Wright CM, Heuckeroth RO (2019) Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annu Rev Physiol 81:235–259
Bliss ES, Whiteside E (2018) The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol 9:900
Wu Y, He H, Cheng Z, Bai Y, Ma X (2019) The role of neuropeptide Y and peptide YY in the development of obesity via gut-brain axis. Curr Protein Pept Sci 20(7):750–758
Zhang X, Grosfeld A, Williams E, Vasiliauskas D, Barretto S, Smith L, Mariadassou M, Philippe C, Devime F, Melchior C, Gourcerol G, Dourmap N, Lapaque N, Larraufie P, Blottiere HM, Herberden C, Gerard P, Rehfeld JF, Ferraris RP, Fritton JC, Ellero-Simatos S, Douard V (2019) Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism. FASEB J (Official publication of the Federation of American Societies for Experimental Biology) 33:7126–7142
Ripken D, van der Wielen N, Wortelboer HM, Meijerink J, Witkamp RF, Hendriks HF (2016) Nutrient-induced glucagon like peptide-1 release is modulated by serotonin. J Nutr Biochem 32:142–150
de Clercq NC, Frissen MN, Groen AK, Nieuwdorp M (2017) Gut microbiota and the gut-brain axis: new insights in the pathophysiology of metabolic syndrome. Psychosom Med 79:874–879
Lach G, Schellekens H, Dinan TG, Cryan JF (2018) Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15:36–59
Klingbeil E, de La Serre CB (2018) Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol 315:R1254–R1260
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108:16050–16055
Meng S, Badrinarain J, Sibley E, Fang R, Hodin R (2001) Thyroid hormone and the d-type cyclins interact in regulating enterocyte gene transcription. J Gastrointest Surg 5:49–55
Virili C, Centanni M (2017) “With a little help from my friends” - The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol Cell Endocrinol 458:39–43
Patil AD (2014) Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab 18:307–309
Biondi B, Cappola AR, Cooper DS (2019) Subclinical hypothyroidism: a review. JAMA 322:153–160
Jacobson DL, Gange SJ, Rose NR, Graham NM (1997) Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243
Virili C, Fallahi P, Antonelli A, Benvenga S, Centanni M (2018) Gut microbiota and Hashimoto’s thyroiditis. Rev Endocr Metab Disord 19:293–300
Alenghat T, Artis D (2014) Epigenomic regulation of host-microbiota interactions. Trends Immunol 35:518–525
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119
Ishaq HM, Mohammad IS, Guo H, Shahzad M, Hou YJ, Ma C, Naseem Z, Wu X, Shi P, Xu J (2017) Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed Pharmacother 95:865–874
Lauritano EC, Bilotta AL, Gabrielli M, Scarpellini E, Lupascu A, Laginestra A, Novi M, Sottili S, Serricchio M, Cammarota G, Gasbarrini G, Pontecorvi A, Gasbarrini A (2007) Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab 92:4180–4184
Zhou L, Li X, Ahmed A, Wu D, Liu L, Qiu J, Yan Y, ** M, **n Y (2014) Gut microbe analysis between hyperthyroid and healthy individuals. Curr Microbiol 69:675–680
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Investig 121:2094–2101
Scheja L, Heeren J (2016) Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol 64:1176–1186
Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, Dalgaard K, Selvaraj M, Gaster M, Lee-Young RS, Febbraio MA, Knauf C, Cani PD, Aberger F, Penninger JM, Pospisilik JA, Esterbauer H (2012) Hedgehog partial agonism drives warburg-like metabolism in muscle and brown fat. Cell 151:414–426
Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jeno P, Beglinger C, Peterli R, Hall MN (2018) Insulin resistance causes inflammation in adipose tissue. J Clin Investig 128:1538–1550
Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA (2009) Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Investig 119:531–539
Sonnenburg JL, Backhed F (2016) Diet-microbiota interactions as moderators of human metabolism. Nature 535:56–64
Zhu Z, Zhu B, Sun Y, Ai C, Wang L, Wen C, Yang J, Song S, Liu X (2018) Sulfated polysaccharide from sea cucumber and its depolymerized derivative prevent obesity in association with modification of gut microbiota in high-fat diet-fed mice. Mol Nutr Food Res 62:e1800446
Zhu A, Chen J, Wu P, Luo M, Zeng Y, Liu Y, Zheng H, Zhang L, Chen Z, Sun Q, Li W, Duan Y, Su D, **ao Z, Duan Z, Zheng S, Bai L, Zhang X, Ju Z, Li Y, Hu R, Pandol SJ, Han YP (2017) Cationic polystyrene resolves nonalcoholic steatohepatitis, obesity, and metabolic disorders by promoting eubiosis of gut microbiota and decreasing endotoxemia. Diabetes 66:2137–2143
Zhuang P, Shou Q, Lu Y, Wang G, Qiu J, Wang J, He L, Chen J, Jiao J, Zhang Y (2017) Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochimica et Biophysica Acta 1863(11):2715–2726
Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT (2018) Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23:41–53, e44
Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA (2008) Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102:11070–11075
Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, Kong C, Wang X, Zhang Y, Qu S, Qin H (2018) Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity (Silver Spring) 26:351–361
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:e9085
Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X, Yu P, Zhao C, Li L, Zhou A, Wang J, Moore JE, Millar BC, Xu J (2010) Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 61:69–78
Mai V, McCrary QM, Sinha R, Glei M (2009) Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J 8:49
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI (2009) A core gut microbiome in obese and lean twins. Nature 457:480–484
Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyoty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW (2011) Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6:e25792
Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO (2015) Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes 2:1–7
Knip M, Siljander H (2016) The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endoc 12:154–167
Yamaguchi Y, Adachi K, Sugiyama T, Shimozato A, Ebi M, Ogasawara N, Funaki Y, Goto C, Sasaki M, Kasugai K (2016) Association of intestinal microbiota with metabolic markers and dietary habits in patients with type 2 diabetes. Digestion 94:66–72
Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E (2017) Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr Diab Rep 17:16
Li G, ** the gut microbiota. Cell Metab 26:672–685, e674
Trent CM, Blaser MJ (2016) Microbially produced acetate: a “Missing Link” in understanding obesity? Cell Metab 24:9–10
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ (2003) The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–11319
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH (2013) Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145:396–406, e391-310
Forbes S, Stafford S, Coope G, Heffron H, Real K, Newman R, Davenport R, Barnes M, Grosse J, Cox H (2015) Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes 64:3763–3771
Remely M, Aumueller E, Merold C, Dworzak S, Hippe B, Zanner J, Pointner A, Brath H, Haslberger AG (2014) Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene 537:85–92
Iepsen EW, Lundgren J, Dirksen C, Jensen JE, Pedersen O, Hansen T, Madsbad S, Holst JJ, Torekov SS (2015) Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes 39:834–841
Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ (2018) The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 315:G53–G65
Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL (2011) Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60:810–818
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286
Bolognini D, Tobin AB, Milligan G, Moss CE (2016) The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 89:388–398
Inoue D, Tsujimoto G, Kimura I (2014) Regulation of energy homeostasis by GPR41. Front Endocrinol (Lausanne) 5:81
Tanoue T, Atarashi K, Honda K (2016) Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16:295–309
Neurath MF, Finotto S, Glimcher LH (2002) The role of Th1/Th2 polarization in mucosal immunity. Nat Med 8:567–573
Wu W, Liu HP, Chen F, Liu H, Cao AT, Yao S, Sun M, Evans-Marin HL, Zhao Y, Zhao Q, Duck LW, Elson CO, Liu Z, Cong Y (2016) Commensal A4 bacteria inhibit intestinal Th2-cell responses through induction of dendritic cell TGF-beta production. Eur J Immunol 46:1162–1167
Weaver JR, Nadler JL, Taylor-Fishwick DA (2015) Interleukin-12 (IL-12)/STAT4 axis is an important element for beta-cell dysfunction induced by inflammatory cytokines. PLoS ONE 10:e0142735
Wang Z, Shen XH, Feng WM, Ye GF, Qiu W, Li B (2016) Analysis of inflammatory mediators in prediabetes and newly diagnosed type 2 diabetes patients. J Diabetes Res 2016:7965317
Lee IK, Hsieh CJ, Chen RF, Yang ZS, Wang L, Chen CM, Liu CF, Huang CH, Lin CY, Chen YH, Yang KD, Liu JW (2013) Increased production of interleukin-4, interleukin-10, and granulocyte-macrophage colony-stimulating factor by type 2 diabetes’ mononuclear cells infected with dengue virus, but not increased intracellular viral multiplication. Biomed Res Int 2013:965853
Schnupf P, Gaboriau-Routhiau V, Gros M, Friedman R, Moya-Nilges M, Nigro G, Cerf-Bensussan N, Sansonetti PJ (2015) Growth and host interaction of mouse segmented filamentous bacteria in vitro. Nature 520:99–103
Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, Vives-Pi M, Powrie J, Tree T, Marchetti P, Huang GC, Gurzov EN, Pujol-Borrell R, Eizirik DL, Peakman M (2011) Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 60:2112–2119
Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA (2003) CD4+CD25+T regulatory cells control anti-islet CD8+T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA 100:10878–10883
Joyce SA, Gahan CG (2016) Bile acid modifications at the microbe-host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu Rev Food Sci Technol 7:313–333
Borghede MK, Schlutter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF (2011) Bile acid malabsorption investigated by selenium-75-homocholic acid taurine ((75)SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med 22:e137–140
Mekjian HS, Phillips SF, Hofmann AF (1971) Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Investig 50:1569–1577
Thaysen EH, Pedersen L (1976) Idiopathic bile acid catharsis. Gut 17:965–970
Ridlon JM, Kang DJ, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Li T, Chiang JY (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983
Schramm C (2018) Bile acids, the microbiome, immunity, and liver tumors. N Engl J Med 379:888–890
Chen J, Thomsen M, Vitetta L (2019) Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J Cell Biochem 120:2713–2720
Parseus A, Sommer N, Sommer F, Caesar R, Molinaro A, Stahlman M, Greiner TU, Perkins R, Backhed F (2017) Microbiota-induced obesity requires farnesoid X receptor. Gut 66:429–437
Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, Kalavagunta PK, Liao J, ** L, Shang J, Li J (2019) Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome 7:9
Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG (2014) Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci USA 111:7421–7426
Sun L, **e C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, **a J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Wang X, Gonzalez FJ, Jiang C (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 24:1919–1929
Guo Z, Zhang J, Wang Z, Ang KY, Huang S, Hou Q, Su X, Qiao J, Zheng Y, Wang L, Koh E, Danliang H, Xu J, Lee YK, Zhang H (2016) Intestinal microbiota distinguish gout patients from healthy humans. Sci Rep 6:20602
Yu Y, Liu Q, Li H, Wen C, He Z (2018) Alterations of the gut microbiome associated with the treatment of hyperuricaemia in male rats. Front Microbiol 9:2233
Garcia-Arroyo FE, Gonzaga G, Munoz-Jimenez I, Blas-Marron MG, Silverio O, Tapia E, Soto V, Ranganathan N, Ranganathan P, Vyas U, Irvin A, Ir D, Robertson CE, Frank DN, Johnson RJ, Sanchez-Lozada LG (2018) Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS ONE 13:e0202901
Yoo JY, Kim SS (2016) Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients 8:173
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG (2013) Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 288:25088–25097
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, R., Li, Y., Li, C., Zheng, D., Chen, P. (2020). Gut Microbiota and Endocrine Disorder. In: Chen, P. (eds) Gut Microbiota and Pathogenesis of Organ Injury. Advances in Experimental Medicine and Biology, vol 1238. Springer, Singapore. https://doi.org/10.1007/978-981-15-2385-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-2385-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2384-7
Online ISBN: 978-981-15-2385-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)