Abstract
Examples of free radical reactions are described for which convincing evidence for quantum-mechanical tunneling has been reported. They include: H-atom abstraction reactions by thermalized methyl radicals and H-atoms in solids (crystalline and glass phases) at low temperatures; radical-pair conversions at 4.2 K; isomerization of hindered aryl radicals and intermolecular H-transfer reactions in fluid solution.
The physical importance and consequences of tunneling have been recognized since the early days of quantum mechanics. The concept of tunneling has become familiar in many fields and has been substantiated for electron transfer reactions, decay of radioactive nuclei (la), electron emission from metals, H-transfer reactions (1), etc.
In 1935 Bell predicted (2) that due to quantum mechanical tunneling, the rate of hydrogen transfer would become almost temperature independent at a very low temperature. Tunneling has been invoked to explain proton transfer data on innumerable occasions (1). However, in many cases the experimental work has been carried out at or even above room temperature where it is difficult to obtain conclusive experimental evidence since the thermally activated process greatly exceeds the contribution from tunneling (3).
Perhaps the most convincing example of tunneling in proton transfer reactions is the one reported by Rentzepis (4) in his study, by picosecond spectroscopy, of the visual process. Upon photolysis (530 nm) rhodopsin undergoes a H+ transfer to yield prelumirhodopsin which appears within 6 ps of excitation and decays with a time constant of ca. 50 ns. The extremely fast formation of prelumirhodopsin at 4 K argues against the cis-trans isomerization process previously thought to be the key step in the visual process. The low temperature data support a H+transfer proceeding by a tunneling mechanism through an activation barrier of ca. 0.7 Kcal/mol. Chemical reactions involving H atom abstraction by free radicals have been studied almost exclusively in the gas phase or in solution above or at room temperature (5,6).
In the solid phase at low temperatures thermally activated process should be suppressed and tunneling should become relatively important. Nevertheless, it was anticipated that H-transfer reactions characterized by activation energies in excess of 5 Kcal/mol at ordinary temperatures, would have negligibly small reaction rates in crystalline or glassy matrices at very low temperatures (7). In an outstanding series of papers, Williams and coworkers have provided convincing evidence that thermal methyl radicals can abstract hydrogen atoms from acetonitrile (8), methyl isocyanide (9), methanol (10) and 3-methylpentane (11) in the solid state at low temperature. The experimental findings could be rationalized on the basis that quantum mechanical tunneling becomes largely responsible for reaction at low temperatures. In these studies, the esr technique gave unequivocal evidence for hydrogen atom abstraction. It was also possible to establish a kinetic coincidence between the two species by monitoring the decay of reactant radicals and the formation of product radicals (12).
The presence of appreciable tunneling in an H-transfer reaction usually leads to: (i) large deuterium kinetic isotope effects, (ii) large differences in effective activation energies for H and D species and (iii) nonlinear Arrhenius plots.
In practical cases, one or more of these phenomena may be observed, depending on the temperature range studied, the accuracy of the data, and the relative importance of the tunneling process.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
For several recent reviews see: a. Caldin, E.F.: 1969, Chem. Rev. 69, pp. 135–156; b. Bell, R.P.: 1974, Chem. Soc. Rev. 3, pp. 513–544; c. Harmony, M.D.: 1972, Chem. Soc. Rev. 1, pp. 211–228; d. Bell, R.P.: 1973, The Proton in Chemisty, eds. Chapman and Hall, London, Chapter 12.
Bell, R.P.: 1935, Proc. Roy. Soc. 148A, pp. 241–250.
Rogne, O.: 1978, Acta. Chem. Scand. 32 Ser. A, pp. 559–563 and references cited therein.
a. Busch, G.E., Applebury, M.L., Lamola, A.A., and Rentzepis, P.M.: 1972, Proc. Natl. Acad. Sci. USA, 69, pp. 2802–2806; b. Sundstrom, V., Rentzepis, P.M., Peters, K.S., and Applebury, M.L.: 1977, Nature (London) 267, pp. 645–646.
Johnston, H.S.: 1960, Adv. Chem. Phys. 3, pp. 131–170.
LeRoy, D.J., Ridley, B.A., and Quickert, K.A.: 1967, Discuss. Faraday Soc. 44, pp. 92–107.
For a review see: Willard, J.E.: 1968, Fundamental Processes in Radiation Chemistry, ed. Ausloos, P., Wiley-Interscience, New York, N.Y. pp. 599–649.
Sprague, E.D. and Williams, F.: 1971, J. Am. Chem. Soc. 93, pp. 787–788.
Wang, J.T. and Williams, F.: 1972, J. Am. Chem. Soc. 94, pp. 2930–2934.
a. Campion, A. and Wiliams, F.: 1972, J. Am. Chem. Soc. 94, pp. 7633–7637; b. Hudson, L., Shiotani, M., and Williams, F.: 1977, Chem. Phys. Lett. 48, pp. 193–196.
a. Sprague, E.D.: 1973, J. Phys. Chem. 77, pp. 2066–2070; b. Neiss, M.A. and Willard, J.E.: 1975, J. Phys. Chem. 79, pp. 783–794; c. Neiss, M.A., Sprague, E.D., and Willard, J.E.: 1975, J. Chem. Phys. 63, pp. 1118–1121.
LeRoy, R.J., Sprague, E.D., and Williams, F.: 1972, J. Phys. Chem. 76, pp. 546–551.
Eckart, C: 1930, Phys. Rev. 35, pp. 1303–1309.
Bell, R.P.: 1959, Trans. Faraday Soc. 54, pp. 1–4.
Ayscough, P.B., Collins, R.G., and Kemp, T.J.: 1966, J. Phys. Chem. 70, pp. 2220–2223.
Bonin, M.A., Tsuji, K., and Williams F.: 1968, Nature (London) 218, pp. 946–948.
Wijnen, M.H.J.: 1954, J. Chem. Phys. 22, pp. 1074–1076.
Sargent, F.P., Bailey, M.G., and Gardy, E.M.: 1974, Can. J. Chem. 52, pp. 2171–2174.
Sprague, E.D., Takeda, K., Wang, J.T., and Williams, F.: 1974, Can. J. Chem. 52, pp. 2840–2844.
Trotman-Dickenson, A.F. and Steacie, E.W.R.: 1951, J. Chem. Phys. 19, pp. 329–336.
Toryiama, K., Nunome, K., and Iwasaki, M.: 1977, J. Am. Chem. Soc. 99, pp. 5823–5824.
a. Toryiama, K. and Iwasaki, M.: 1978, J. Phys. Chem. 82, pp. 2056–2061; b. Iwasaki, M., Toryiama, K., Muto, H., and Nunome, K.: 1978, Chem. Phys. Lett. 56, pp. 494–496.
Timm, D. and Willard, J.E.: 1969, J. Phys. Chem. 73, pp. 2403–2408.
Aditya, S., Wilkey, D.D., Wang, H.Y., and Willard, J.E.: 1979, J. Phys. Chem. 83, pp. 599–605. See also: Wilkey, D.D. and Willard, J.E.: 1976, J. Chem. Phys. 64, pp. 3976–3977.
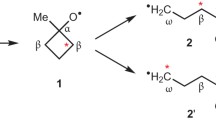
a. Brunton, G., Griller, D., Barclay, L.R.C., and Ingold, K.U.: 1976, J. Am. Chem. Soc. 98, pp. 6803–6811; b. Brunton, G., Gray, J.A., Griller, D., Barclay, L.R.C., and Ingold, K.U.: 1978, J. Am. Chem. Soc. 100, pp. 4197–4200.
Malatesta, V. and Ingold, K.U.: J. Am. Chem. Soc., submitted.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1981 D. Reidel Publishing Company
About this paper
Cite this paper
Malatesta, V., Ingold, K.U., Chatgilialoglu, C. (1981). Quantum Mechanical Tunneling in Free Radical Reactions. In: Capellos, C., Walker, R.F. (eds) Fast Reactions in Energetic Systems. NATO Advanced Study Institutes Series, vol 71. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-8511-7_29
Download citation
DOI: https://doi.org/10.1007/978-94-009-8511-7_29
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-009-8513-1
Online ISBN: 978-94-009-8511-7
eBook Packages: Springer Book Archive