Summary
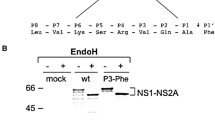
Dengue type 4 (DEN4) and other flaviviruses employ host and viral proteases for polyprotein processing. Most proteolytic cleavages in the DEN4 nonstructural protein (NS) region are mediated by the viral NS2B-NS3 protease. The N-terminal third of NS3, containing sequences homologous to serine protease active sites, is the protease domain. To determine required sequences in NS2B, deletions were introduced into DEN4 NS2B-30%NS3 cDNA and the expressed polyproteins assayed for self-cleavage. A 40 amino acid segment within NS2B was essential. Sequence analysis of NS2B predicts that this segment constitutes a hydrophilic domain surrounded by hydrophobic regions. Hydophobicity profiles of other flavivirus NS2Bs show similar patterns. Cleavage of DEN4 NS1-NS2A requires an octapeptide sequence at the NS1 C terminus and downstream NS2A. Comparison of the analogous octapeptide sequences among flaviviruses indicates a consensus cleavage sequence of (P8)/Met/Leu-Val-Xaa-Ser-Xaa-Val-Ala(P1), where Xaa are non- conserved amino acids. The effects on cleavage of amino acid substitutions in this consensus sequence were analyzed. Most substitutions of the conserved residues interfered with cleavage, whereas substitutions of non-conserved residues had little or no effect. These findings indicate that the responsible enzyme recognizes well-defined sequences at the cleavage site.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Falgout B, Chanock R, Lai C-J (1989) Proper processing of dengue virus nonstructural glycoprotein NS1 requires the N-terminal hydrophobic signal sequence and the downstream nonstructural protein NS2A. J Virol 63: 1852–1860
Falgout B, Lai C-J (1990) Synthesis of dengue virus nonstructural protein NS1 requires the N-terminal signal and the downstream nonstructural protein NS2A. In: Brinton MA, Heinz FX (eds) New aspects of positive-strand RNA viruses. Americal Society for Microbiology, Washington, pp 192–195
Hori H, Lai C-J (1990) Cleavage of dengue virus NS1-NS2A requires an octapeptide sequence at the C-terminus of NS1. J Virol 64: 4573–4577
Pethel M, Falgout B, Lai C-J (1992) Mutational analysis of the octapeptide sequence motif at the NS1-NS2A cleavage junction of dengue type 4 virus. J Virol 66: 7225–7231
Bazan JF, Flettrick RJ (1989) Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology 171: 637 - 639
Gorbalenya AE, Donchenko AP, Koonin EV, Blinov VM (1989) N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res 17: 3889–3897
Falgout B, Pethel M, Zhang Y-M, Lai C-J (1991) Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol 65: 2467–2475
Cahour A, Falgout B, Lai C-J (1992) Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B-NS3, whereas NS4A/NS4B may be processed by a cellular protease. J Virol 66: 1535–1542
Preuschat F, Yao C-W, Strauss JH (1990) In vitro processing of dengue 2 nonstructural proteins NS2A, NS2B and NS3. J Virol 64: 4364–4374
Chambers TJ, Weir RC, Grakoui A, McCourt DW, Bazan JF, Fletterick RJ, Rice CM (1990) Evidence that the N-terminal domain of yellow fever virus NS3 is a serine protease responsible for site-specific cleavage in the viral polyprotein. Proc Natl Acad Sci USA 87: 8898–8902
Falgout B, Miller R, Lai C-J (1993) Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of domains required for NS2B-NS3 protease activity. J Virol 67: 2034–2042
Arias CF, Preugschat F, Strauss JH (1993) Dengue 2 virus NS2B and NS3 form a stable complex that can cleave NS3 within the helicase domain. Virology 193: 888–899
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1994 Springer-Verlag
About this paper
Cite this paper
Lai, CJ., Pethel, M., Jan, L.R., Kawano, H., Cahour, A., Falgout, B. (1994). Processing of dengue type 4 and other flavivirus nonstructural proteins. In: Brinton, M.A., Calisher, C.H., Rueckert, R. (eds) Positive-Strand RNA Viruses. Archives of Virology Supplementum, vol 9. Springer, Vienna. https://doi.org/10.1007/978-3-7091-9326-6_36
Download citation
DOI: https://doi.org/10.1007/978-3-7091-9326-6_36
Publisher Name: Springer, Vienna
Print ISBN: 978-3-211-82522-8
Online ISBN: 978-3-7091-9326-6
eBook Packages: Springer Book Archive