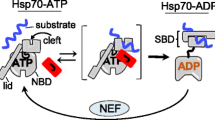
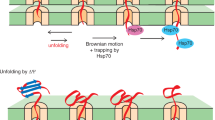
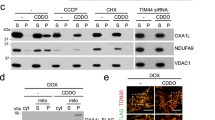
Abstract
Most proteins destined for cell organelles are synthesized on cytosolic polysomes and must cross one or more organelle membranes to reach their functional destination (Wickner and Lodish, 1985). With mitochondria, over 95% of the proteins are made as precursor proteins in the cytosol and are mainly post-translationally imported into the four mitochondrial subcompartments (outer membrane, intermembrane space, inner membrane, and matrix) (Attardi and Schatz, 1988; Hartl and Neupert, 1990; Pfanner and Neupert, 1990). Heat shock proteins (hsps) were found to play an important role at various stages of mitochondrial protein import. This includes the maintenance of a transport-competent conformation of precursor proteins by cytosolic hsp70s (Deshaies etal., 1988; Murakami etal., 1988; Pfanner etal., 1990), the involvement of mitochondrial HSP70 in translocation of precursor proteins through contact sites between mitochondrial outer and inner membranes (Kang etal., 1990), and the refolding of imported proteins at HSP60 in the mitochondrial matrix (Ostermann etal., 1989).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Attardi, G and Schatz, G, (1988) Biogenesis of mitochondria. Ann. Rev. Cell Biol., 4: 289–333.
Chen, W-J and Douglas, MG, (1987) Phosphodiester bond cleavage outside mitochondria is required for the completion of protein import into the mitochondrial matrix. Cell, 49: 651–658.
Cheng, MY, Hartl, F-U, Martin, J, Pollock, RA Kalousek, F, Neupert, W, Hallberg, EM, Hallberg, RL and Horwich, AL, (1989) Mitochondrial heat- shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature, 337: 620–625.
Deshaies, RJ, Koch, BD, Werner-Washburne, M, Craig, E. A and Schekman, R, (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature, 332: 800–805.
Ellis, RJ and Hemmingsen, SM, (1989) Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem. Sci., 14: 339–342.
Goloubinoff, P, Christeller, JT, Gatenby, AA and Lorimer, GH, (1989) Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins ana Mg-ATP. Nature, 342: 884–889.
Hartl, F-U and Neupert, W, (1990) Protein sorting to mitochondria: evolutionaiy conservations of folding and assembly. Science, 247: 930–938.
Kang, P-J, Ostermann, J, Shilling, J, Neupert, W, Craig, EA and Pfanner, N, Hsp70 in the mitochondrial matrix is required lor translocation and folding of precursor proteins. Submitted.
Kawasaki, Y, Wada, C and Yura, T, (1990) Roles of Escherichia coli heat shock proteins DnaK, DnaJ and GrpE in mini-F plasmid replication. Mol. Gen. Genet., 220: 277–282.
Mahlke, K, Pfanner, N, Martin, J, Horwich, AL, Hartl, F-U and Neupert, W, Sorting pathways of mitochondrial inner membrane proteins. Eur. J. Bioehem., in press.
Meyer, DI, ( 1988) Preprotein conformation: the year’s major theme in translocation studies. Trends Biochem. Sci., 13: 471–474.
Ostermann, J, Horwich, AL, Neupert, W and Hartl, F-U, (1989) Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature, 341: 125–130.
Pelham, H, (1988) Heat shock proteins: coming in from the cold. Nature, 332: 776–777.
Pfanner, N and Neupert, W, (1985) Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. EMBO J., 4: 2819–2825.
Pfanner, N and Neupert, W, (1990) Hie mitochondrial protein import apparatus. Annu. Rev. Biochem., 59: 331–353.
Pfanner, N, Tropschug, M and Neupert, W, (1987) Mitochondrial protein import: nucleoside triphosphates are involved in conferring import- competence to precursors. Cell, 49: 815–823.
Pfanner, N, Pfaller, R, Kleene, R, Ito, M, Tropschug, M and Neupert, W, (1988) Role of ATP in mitochondrial protein import: conformational alteration of a precursor protein can substitute for ATP requirement. J. Biol. Chem., 263: 4049–4051.
Pfanner, N, Rassow, J, Guiard, B, Sdllner, T, Hartl, F-U and Neupert, W, Energy requirements for unfolding and membrane translocation of precursor proteins during import into mitochondria. J. Biol. Chem., in press.
Randall, SK and Shore, GC, (1989) Import of a mutant mitochondrial precursor fails to respond to stimulation by a cytosolic factor. FEBS Lett., 250: 561–564.
Rassow, J, Hartl, F-U, Guiard, B, Pfanner, N and Neupert, W, Polypeptides traverse the mitochondrial envelope in an extended state. Submitted.
Rothman, JE, (1989) Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell, 59: 591–601.
Schleyer, M and Neupert, W, (1985) Transport of proteins into mitochondria: transnational intermediates spanning contact sites between outer and inner membranes. Cell, 43: 339–350.
Verner, K and Schatz, G, (1987) Import of an incompletely folded precursor protein into isolated mitochondria requires an energized inner membrane, but no added ATP. EMBO J., 6: 2449–2456.
Vogel, JP, Misra, LM and Rose, MD, (1990) Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol., 110: 1885–1895.
Wickner, WT and Lodish, HF, (1985) Multiple mechanisms of protein insertion into and across membranes. Science, 230: 400–407.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1991 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Pfanner, N. (1991). Role of Heat Shock Proteins in Mitochondrial Protein Import. In: Maresca, B., Lindquist, S. (eds) Heat Shock. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-76679-4_19
Download citation
DOI: https://doi.org/10.1007/978-3-642-76679-4_19
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-76681-7
Online ISBN: 978-3-642-76679-4
eBook Packages: Springer Book Archive